Next to mangos and bananas, pineapples are the third most consumed fruit worldwide. Diseases of pineapple [Ananas comosus (L.) Merr.] are therefore economically important in the production of fresh and canned fruit products. The pink disease of pineapple represents one of the most perplexing problems of the pineapple canned-fruit industry. The disease is virtually asymptomatic in the field. Pink disease symptoms are primarily recognized when the diseased fruit is canned. The heating process required for canning causes the formation of red and rusty red colored fruit slices that were processed from diseased fruits. Such blemished canned fruits are not marketable. Pink disease represents one of the most important and challenging diseases of pineapple.
History
Pink disease was originally described in 1915 in Hawaii (6). The pathogen responsible for causing pink disease remained obscure and the nature of the pink color formation of the pineapple fruit tissue was not understood. A myriad of bacteria associated with the pineapple plant, many of which originated from the surrounding soil, made identifying the primary cause of the disease extremely difficult. The biochemical basis of the disease was thought to be complex and difficult to elucidate, and was therefore left uncharacterized. Attempts at identifying the pathogen led to implicating several distinct bacteria as the causal agents of pink disease (5,10). Gluconobacter oxydans, Acetobacter aceti, and Erwinia herbicola were the prominent suspected species. These organisms have been characterized with regard to their nutritional requirements and optimal growth properties (4), and are classified in distinct families: Acetobacteriaceae (as a member of the alpha-proteobacteria) and Enterobacteriaceae (as a member of the beta-proteobacteria). No members of the Acetobacteriaceae are known to be plant pathogens, thus neither G. oxydans nor A. aceti have been previously reported as specific plant pathogens. Erwinia herbicola is a member of the Enterobacteriaceae, a family that contains certain known plant pathogens in the genus Brenneria, Erwinia, and Pantoea. Species classified as Erwinia herbicola, also known as Enterobacter agglomerans, were previously found to vary phenotypically and generally found to comprise a heterogeneous collection of yellow and semi-yellow pigmented bacteria that were mainly saprophytic.
Using molecular genetics approaches, the causal agent of pink disease was identified recently as Pantoea citrea (2). Also, the biochemical pathway leading to the pink coloration associated with pink disease was elucidated (3,8,9).
Symptoms and Biochemical Cause of Pink Coloration

Fig. 1. Pink disease symptoms on pineapple fruit slices originating from a canned product. Healthy fruit (top), and diseased fruit (bottom). |
|
Pink disease symptoms are difficult to observe in the field since outward symptoms are not apparent. Infections of the foliage are not usually found. Under severe invasion of the fruit by P. citrea, a translucent appearance of the sub-dermal fruit tissue occasionally can be observed. The most common appearance of symptoms occurs when infected fruit preparations are heated as a result of the canning process. Heating causes the formation of red to rusty brown coloration of the usually golden yellow tissue (Fig. 1).
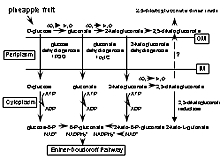
The biochemical basis of the red color formation in pineapple juice and fruit can be attributed to the ability of P. citrea to oxidize glucose into 2,5-diketogluconic acid via a two step conversion as shown in the biochemical pathway illustrated in Fig. 2. Glucose is converted to gluconate by gluconate dehydrogenase. There are two gluconate dehydrogenases produced by P. citrea. The first enzyme encoded by gdhA is common among many different bacteria, while the second enzyme encoded by the gdhB gene is relatively distinct and specific to P. citrea (9). Gluconate is further oxidized into 2-ketogluconate by this enzyme. The product of this reaction is oxidized into 2,5-diketogluconate by 2-ketogluconate dehydrogenase. The heating of this end-product results in the formation of a dark red color, presumably due to the condensation and the formation of a double bond linking the two 2,5-diketogluconate molecules as a dimer. The dark red reaction is shown in Fig. 3 when the naturally present D-glucose in pineapple juice is oxidized into 2,5-diketogluconate via 2-ketogluconate dehydrogenase. No other sugar substrates lead to the formation of 2,5-diketogluconate.
 |
|
 |
|
Fig. 2. Biochemical pathway converting glucose to 2,5-diketogluconate via oxidative reactions. The end product is secreted into the environment where it forms a dimerized chromogen. An alternative pathway converting glucose to phosphogluconate production via several ATP hydrolysis steps leads to the Entner-Doudoroff pathway. |
|
Fig. 3. Pink disease color reaction in pineapple juice that promoted the growth of wild-type P. citrea (third tube from left) and mutant strains defective in their ability to convert glucose into 2,5-diketogluconate (tubes 4, 5, and 6 from left). Tubes 1 and 2 are plain juice and juice containing heat-killed P. citrea as the inoculum. |
Disease Cycle
| |

Fig. 4. Example of an immature pineapple fruit bearing blossoms that are commonly visited by flying insects.
|
The disease cycle of pink disease remains unresolved. Pantoea citrea appears to gain access into the fruit via the numerous florets that grow out from the growing fruit (Fig. 4). Because there is a relatively consistent correlation between spraying of insecticides during the blooming season and the concomitant reduction of pink disease, insects are thought to play a role in disseminating the pathogen from flower to flower. Once entry into the fruit occurs, the pathogen colonizes the intercellular portions of the fruit tissue. With time, infected tissues will show a moderate translucent appearance (water soaking) but no soft-rotting symptoms. The infected tissues become colored upon canning. Pantoea citrea presumably multiplies in the flowers and becomes the source of inoculum vectored by the insects. Because pineapple plants are propagated on a year-round basis, transmission of P. citrea from field-to-field sustains pink disease propagation.
The Pathogen
Pantoea citrea is a Gram-negative, facultative anaerobic, non-spore forming, bacilliform bacterium with physiological and biochemical as well as 16S rDNA features corresponding to those of the Enterobacteriaceae. On nutrient agar and trypticase soy agar, the colonies are entire, smooth, glistening, translucent, but not mucoid. The colonies become taupe in color. Pantoea citrea grows readily in pineapple juice as well as in fresh pineapple fruit tissue. Unlike other Pantoea species, P. citrea is unable to utilize citrate or tartrate. Besides the genetic makeup that causes the pink disease reaction in the pineapple fruit, the bacterium elicits the hypersensitive response in tobacco (2). Many strains harbor pUCD5000, a small plasmid containing genes that help promote the development of pink coloration (7).
The pathogen is amenable to genetic manipulation and is compatible with many plasmid vectors used as molecular biological tools. The sequence of the entire genome is forthcoming and should shed a complete picture on the organization of operons and genes involved in causing the pink disease in pineapple.
Control
| |

Fig. 5. Application of insecticide by means a long arm boom sprayer extending both sides of supplier truck. Insecticide is applied multiple times during the flowering season. |
|
Current methods of controlling the disease are relatively expensive since multiple applications of insecticides are necessary to maintain low levels of pink disease incidence (Fig. 5). Usually, insecticides are applied during the early through late blooming periods of the pineapple fruit. Insects generally appear at night and roam the ripening fields. Insecticide use is predicated on the assumption that one or more flying insects vector the pink disease pathogen from flower to flower. Although there is no experimental evidence attributing insects directly with the transmission of P. citrea to the fruit, the high correlation of higher pink disease incidence with lowered application of insecticides tend to suggest that this assumption is correct.
Plant breeding for resistance to pink disease has shown some promise. Crosses between the wild-type resistant varieties with the horticulturally acceptable varieties such as Smooth Cayenne cultivars are currently being screened to develop successful resistance.
Plant genetic engineering strategies are also being considered. Genes used to lower the substrate that leads to 2,5-diketogluconate formation and genes used to inhibit the growth of P. citrea in fruit tissue are some examples that can be incorporated in the transgenic pineapple.
Biological control methods also have been assessed. Several bacterial species that are antagonistic to P. citrea have been tested in the laboratory and in the field (Fig. 6) (1). The most promising biocontrol isolates, such as Bacillus gordonae 2061R (1), further reduced disease incidence in combination with insecticides. From a practical view point, the requirement of relatively large fermentation facilities to produce and process large quantities of bacteria is a key limiting factor. Production, supply, maintenance, and trained labor are needed to continually produce the biocontrol agent. This end of the biocontrol program is not cost effective when compared with insecticides. Outside suppliers of the biocontrol agent would help alleviate some of the production cost. However, for one pineapple producing company alone, more than 60 square miles (15,540 hectares) of pineapple are propagated year round. Hence, the application of a biocontrol agent (e.g., at the rate of 1 kg of biocontrol inoculum [wet packed weight] per hectare requires 50 liters of culture medium) to such a vast area is perceived as economically unfeasible.
| |

Fig. 6. Manual spray application of Bacillus gordniae 2061R inoculum covering five 25 acre-replicates during optimum flowering period. Two applications, two weeks apart, were conducted. Each fruit was harvested near maturity and the core was tested for pink disease by heating. |
|
Literature Cited
1. Cha, J.-S., Ducusin, A. R., Macion, E. A., Lucas, L. N., Hubbard C. H., and Kado, C. I. 1994. Biological control of the pink disease of pineapple using bacterial epistasis. Mol. Ecol. 3:609.
2. Cha, J.-S., Pujol, C., Ducusin, A. R., Macion, E. A., Hubbard, C. H., and Kado, C. I. 1997. Studies on Panotea citrea, the causal agent of pink disease of pineapple. J. Phytopathol. 145:313-319.
3. Cha, J.-S., Pujol, C., and Kado, C. I. 1997. Identification and characterization of a Pantoea citrea gene encoding glucose dehydrogenase that is essential for causing pink disease of pineapple. Appl. Environ. Microbiol. 63:71-76.
4. Cho, J. J., Hayward, A. C., and Rohrbach, K. G. 1980. Nutritional requirements and biochemical activities of pineapple pink disease bacterial strains from Hawaii. Anton. Van Leeuwenhoek 46:191-204.
5. Kontaxis, D. G., and Hayward, A. C. 1978. The pathogen and symptomatology of pink disase of pineapple fruit in the Philippines. Plant Dis. Reptr. 62:446-450.
6. Lyon, H. L. 1915. a survey of the pineapple problems. Hawaii. Plant. Rec. 13:125-139.
7. Pujol, C. J., and Kado, C. I. 1998. Characterization of pUCD5000 involved in pink disease color formation by Pantoea citrea. Plasmid 40:169-171.
8. Pujol, C. J., and Kado, C. I. 2000. Genetic and biochemical characterization of the pathway in Pantoea citrea leading to pink disease of pineapple. J. Bacteriol. 182:2230-2237.
9. Pujol, C. J., and Kado, C. I. 1999. gdhB, a gene encoding a second quinoprotein glucose dehydrogenase in Pantoea citrea, is required for pink disease of pineapple. Microbiol. 145:1217-1226.
10. Rohrbach, K. G., and Pfeiffer, J. B. 1976. The interaction of four bacteria causing pink disease of pineapple with several pineapple cultivars. Phytopathology 66:396-399.
