Crown gall
Agrobacterium tumefaciens
Members of 93 families of plants.
Author
Clarence I. Kado
University of California, Davis

Crown gall on Euonymus caused by
Agrobacterium tumefaciens. (Courtesy R.L. Forster)
Symptoms and signs
Crown gall is identified by overgrowths appearing as galls on roots and at the base or "crown" of woody plants such as pome (e.g., apple, pear) and stone (e.g., cherry, apricot) fruit and nut (e.g., almond, walnut) trees (Figure 1). Crown galls are also formed on ornamental woody crops such as roses, Marguerite daisies, and
Chrysanthemum spp. as well as on vines and canes such as grapevines (Figure 2) and raspberries. Marguerite daisies, chrysanthemums and grapevines can become infected systemically. Occasionally, galls have been observed on field crops such as cotton, sugar beets, tomatoes, beans (Figure 3) and alfalfa (Figure 4), but the disease does not impact such crops economically. Crown gall is caused by
Agrobacterium tumefaciens, a Gram-negative, bacilliform bacterium that is normally associated with the roots of many different plants in the field. This bacterium can survive in the free-living state in many soils with good aeration such as sandy loams where crown gall diseased plants have grown. The bacterium can also survive on the surface of roots (rhizoplane) of many orchard weeds.

Figure 1 |

Figure 2 |

Figure 3 |

Figure 4 |
| |
Plants representing over 93 plant families are susceptible to crown gall as judged by experimental inoculations. Owing to their high susceptibility to crown gall, plants such as Jimson weed
(Datura stramonium) and sunflower (Helianthus annuus) are used as assay hosts for testing the degree of virulence of
A. tumefaciens. Also,
Kalanchoë daigmontiana (also known as
Bryophyllum) is used for assaying
A. tumefaciens, but the plant is less sensitive than
Datura.
Pathogen Biology
Agrobacterium tumefaciens is a rhizoplane bacterium whose characteristics are Gram-negative, strictly aerobic, bacilliform rods measuring 1 x 3 µm, and whose nutritional requirements are non-fastidious. The rods bear flagella that are arranged subpolarly around the cylindrical circumference of the cell, referred as circumthecal flagellation (Figure 5). When
A. tumefaciens cells perceive plant phenolic compounds, the virulence genes that are located in the resident Ti (tumor-inducing) plasmid are expressed, resulting in the formation of a long flexuous filament called the T pilus (see next section). The activation of VirA also shuts off motility of the circumthecal flagella, presumably when
A. tumefaciens cells attach to plant cells. Attachment to the plant cells is a prerequisite for initiating the transfer of the T-DNA into the plant cell. Both the circumthecal flagella and the T pilus play an essential role in virulence, presumably by bringing the bacterial cell to its target followed by attachment to the plant host, respectively.
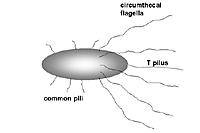
Figure 5 |
Agrobacterium tumefaciens biotypes and biovars
Based on some distinct phenotypic differences,
A. tumefaciens isolates were originally classified into three biotypes or biovars (biotype I, II and III; biovar 1, 2, and 3). Biotype I or biovar 1 strains produce 3-ketosugars and usually have wide host ranges. Biotype II or biovar 2 strains mainly classify as the hairy root-forming organism,
A. rhizogenes. Biotype III or biovar 3 isolates are mainly confined to grapevines, prefer L-tartaric acid over glucose and produce polygalacturonase. Because grapevine isolates formed a distinct group verified by DNA homology studies and were frequently limited in host-range to grapevines, biovar 3 strains have been reclassified into one species,
A. vitis. Agrobacterium rubi strains infect canes of the genus
Rubus, representing blackberry and raspberry.
Ti plasmid and virulence genes
Experimental inoculation of an assay host plant such as Jimson weed (Datura stramonium) results in tumor formation within two weeks (Figure 6). Virulence and the host-range of
A. tumefaciens are conferred by a large extrachromosomal DNA element designated as the Ti plasmid (for tumor-inducing) that resides in all virulent strains of this pathogen. The Ti plasmid is approximately 200 kilobases in length and is comprised of a covalently closed, double-stranded DNA circular molecule.
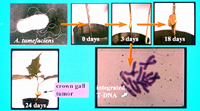
Figure 6 |
Upon recognition of plant signals in the form of dimethoxyl phenolic compounds released from plant wounds, virulence gene,
virA, encodes histidine kinase (VirA) that phosphorylates the response regulator (VirG), which in turn transcriptionally activates the remaining
vir genes of the
vir regulon. The products of the
vir genes carry out the processing of the T-DNA and functions for T-DNA transfer to the plant cell. Also, perception of the plant phenolic compounds by VirA switches off
A. tumefaciens cell motility. In addition, certain chromosomal genes (e.g.,
chv,
att genes) of
A. tumefaciens are also involved in virulence. Most of them have a role in the attachment of
A. tumefaciens to plant cells, in promoting signal molecule recognition, and in the regulation of certain
vir and T-DNA genes.
T-DNA transfer from
A. tumefaciens to plants is aided by a long flexuous appendage known as the T pilus. The T-DNA is delivered as a single-stranded DNA molecule coupled to a VirD2 protein into the plant cell. The T-DNA is integrated into the plant chromosome as evidenced by in situ hybridization using the T-DNA as the probe (Figure 6). The T-DNA genes are expressed by plant transcriptional machinery.
The products of the T-DNA genes catalyze the formation of auxin, cytokinin and opines. Profuse production of auxin and cytokinin in the transformed cells results in abnormal cell division, cell enlargement, and uncontrolled growth of the infected plant tissues. Opines are utilized as specific nitrogen and carbon sources by
A. tumefaciens. The opines produced by the transformed plant cells provide unique nutritional sources for the pathogen, and certain opines promote transfer of the Ti plasmid between
Agrobacterium strains. Certain opines such as octopine and agrocinopine serve as inducers of the Ti plasmid conjugative transfer system.
Injury to young tissues leads to infection
Wounds made by cultivating practices include accidental, localized injuries caused by mowing and disking machines for weed removal in orchards and vineyards. The wounds are an open invitation for the initiation of the crown gall disease of the roots and the base of trees and vines. Also, tissues injured by frost are prone to infection by
A. tumefaciens. In plants where
A. tumefaciens resides systemically throughout the plant, such as in Marguerite daisy, grapevines and chrysanthemum, frost injury can cause a linear or confluent array of small tumors along the vascular system of the infected plant (Figure 2). Galls on subterranean parts originated from initial planting of diseased or infected stock (Figure 7). Wounds made by grafting and pruning before transplanting are also susceptible to crown gall infection.

Figure 2 |

Figure 7 |
Crown gall tumors are often fleshy and easily detached
Fresh crown galls are relatively sturdy and hard. As these galls age beyond one year, they appear convoluted with cavities and become friable. Insects such as earwigs commonly reside within these cavities. It is difficult to isolate
A. tumefaciens from aged galls when most of the diseased plant tissue is dead. Aged galls are easily detached. Fresh galls are the best sources of this pathogenic bacterium.
Crown gall causes stunting of growth
Both loss of yield and stunting of growth may occur when seedlings or young cuttings are infected in the early stages of plant growth. The lack of vigor, reduction in foliage, and water stress are associated with chronically diseased root systems. When more mature tree crops become infected, secondary growths will appear from the root systems near the trunk. These "suckers" are a good sign that the root system is infected.
Disease Cycle and Epidemiology
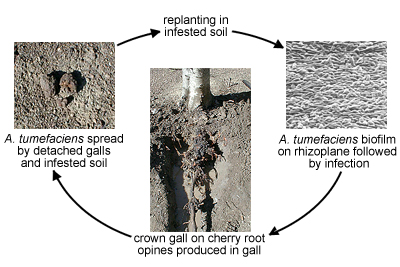
Disease cycle of crown gall caused by
Agrobacterium tumefaciens (Courtesy C. Kado). Disease Cycle and Epidemiology
Agrobacterium tumefaciens naturally resides on the rhizoplane of woody and herbaceous weeds. Its presence in soils originates from galls that were broken or sloughed off from infected plants during cultivation practices or disseminated as infected plant material. Irrigation aids in further dissemination of the
A. tumefaciens bacterial cells.Agrobacterium tumefaciens is also spread by infected and infested planting stocks originating as nursery stock from uncertified sources. Secondary spread then occurs through pruning and cultivation equipment, particularly when galls are removed manually with the same cutting tools used in pruning. Tilling equipment can be contaminated by cutting through galls at or near the base of trunks of infected trees. Rogueing (removal) of infected trees and replanting in the same spot where the infected tree had grown is poor practice because sloughed off galls serve as sources of abundant populations of
A. tumefaciens.
A. tumefaciens enter a plant wound, disease will be more severe and a large gall will be seen rapidly growing at the infection site (Figure 6). Plants that are systemically infected (such as grapevines) will harbor
A. tumefaciens for extended periods of time in the absence of overt symptoms. Tissue injuries induced by accidental wounds made during cultivation practices or by frost will elicit new infections with the appearance of numerous galls along the vascular system of the host (Figure 4).

Figure 4 |

Figure 6 |
Crown gall Management
Preplanting Management Options
Planting stocks. Visual examination for crown gall tumors has been the conventional primary screen for diseased material. The method is limited for complete disease control because
A. tumefaciens can reside on the rhizoplane and systemically in certain host plants such as grapevines, chrysanthemums, and marguerite daisy.
Site selection. Fields that have grown cereal crops for a long period are favored as crown gall-free sites. Fields previously used for growing fruit and nut crops can remain infested with
A. tumefaciens. Certain weeds such as morning glory (Ipomoea leptphylla) can serve as natural hosts of
A. tumefaciens and therefore perpetuate the survival of this pathogen in field soils.
Crop rotation. A crop rotation program employing cereal crops followed by green manuring helps reduce the population size of
A. tumefaciens.
Chemical eradicants. Eradication of crown gall using creosote-based compounds, copper-based solutions, and strong oxidants such as sodium hypochlorite are transiently effective. The chemical eradicant application procedure is labor intensive and therefore costly both monetarily and to the environment. The superficial treatments are ineffective against systemically infected plants. Generally, chemicals are rarely used for control of crown gall.
Biological control. Certain strains of
A. tumefaciens are sensitive to the antibiotic agrocin produced by
A. radiobacter, a closely related soil-borne bacterium that does not infect plants. An example of an antibiotic produced is Agrocin-84, which is an analog of the opine agrocinopine A. Agrocinopine A is produced in crown gall tumors induced by
A. tumefaciens strains whose Ti plasmid encodes for nopaline and agrocinopine A. Agrocin-84 mimics agrocinopine A and therefore is taken up by the same transport system used by
A. tumefaciens to utilize agrocinopine A. Inside the
A. tumefaciens cell, the antibiotic Agrocin-84 inhibits DNA replication and cellular growth.
Plants are protected against Agrocin-84 sensitive strains of the pathogen by dipping the root system into a suspension of
A. radiobacter K84 before planting in infested fields. Biological control of crown gall has been a very effective method to control crown gall in several locations. In many other regions where agrocin-insensitive strains of
A. tumefaciens (strains that do not acquire agrocinopine A) reside, this biological control strategy is ineffective.
Genetically engineering. Transgenic crop plants harboring one or more unique genes tailored to protect the plant from crown gall have been developed. Genes encoding products that degrade or inactivate the T-DNA strand complex when it enters the host cell, that prevent the expression of T-DNA genes encoding indoleacetic acid and cytokinin biosynthesis, and that prevent
A. tumefaciens attachment to its target are some examples currently being tested. Biotechnology companies, such as DNA Plant Technology
(Oakland, CA), are applying sense strand messenger RNA or small-interfering RNAs to develop crown gall resistant fruit and nut crops.
Management in Established Fields
Trees in fruit and nut orchards can be maintained over long periods if the trees became infected at maturity. Diseased trees will bear crop, but with age the trees will become unthrifty and suffer dehydration as their root system becomes progressively infected. The removal of infected trees and vines is costly in loss in time and in money. Annual row crops such as sugar beets and cotton occasionally will have a few plants with crown gall, but the disease is considered of low economic importance. Perennial field crops such as alfalfa will occasionally become infected with
A. tumefaciens where the organism is spread by the mowing equipment. Usually, rogueing of the diseased plants is sufficient to minimize further spread of crown gall.
Historical Significance
Agrobacterium tumefaciens was originally named
Bacterium tumefaciens by Cavara in 1897. In 1907, the name was changed to
Phytomonas tumefaciens (Smith & Townsend) Bergey et al.) by E. F. Smith, which was subsequently changed to
A. tumefaciens, the taxon name of current use. This organism was first isolated from grapevines in 1897 by Fridiano Cavara at the Laboratorio di Botanica del Recherci Instituto Forestale di Vallom Drosa in Naples, Italy. In 1907, E. F. Smith and C. O. Townsend in the United States isolated the bacterium from chrysanthemum galls.
As interest grew in determining how
A. tumefaciens caused crown gall, a large number of
A. tumefaciens strains were isolated and characterized. By the late 1940s, it became apparent that a novel tumor-inducing factor or principle was responsible for initiating the crown gall tumor disease. Although various factors including the secretion of phytohormones, the transmission of bacteriophage, and DNA were implicated, the presence of an extrachromosomal element in all virulent strains of
A. tumefaciens was discovered. This important discovery led to extensive studies on the extrachromosomal element termed the Ti plasmid (Ti for tumor-inducing). That a specific portion of the Ti plasmid was transferred and incorporated into the genome of the host plant opened the way to incorporate novel genes into plants.Agrobacterium tumefaciens is considered the organism that launched plant genetic enginee
ring.
s
Burr, T. J. and L. Otten. 1999. Crown gall of grape vine: biology and disease management. Annu. Rev. Phytopathology 37:9004.
D’Arcy, C.J. and D.M. Eastburn. 2003. Crown gall. Plants, Pathogens, and People website. Includes animation in disease cycle.http://www.ppp.uiuc.edu
Ferguson, G. C., and J. A. Heinemann. 2002. Recent history of trans-kingdom conjugation. Pages 3-17. In: Horizontal Gene Transfer. M. Syvanen and C. I. Kado, eds. Academic Press, London.
Kado, C. I. 1976. The tumor-inducing substance of
Agrobacterium tumefaciens. Annu. Rev. Phytopathol. 14:265-308.
Kado, C. I. 2002. Crown gall tumors. Pages 1-3. in: Encyclopedia of Genetics. S. Brenner and J. H. Miller, eds. Academic Press, San Diego, CA.
McGuire, R. G., P. Rodriguez-Palenzuela, A. Collmer and T. J. Burr. 1991. Polygacturonase production by
Agrobacterium tumefaciens biovar 3. Appl. Environ. Microbiol. 57:660-664.
Ophel, K., and A. Kerr. 1990.Agrobacterium vitis, new species for strains of
Agrobacterium biovar 3 from grapevines. Intl. J. Syst. Bacteriol. 40:236-241.
Zaenen, I., N. Van Larebeke, H. Teuchy, M. Van Montagu and J. Schell. 1974. Supercoiled circular DNA in crown-gall inducing
Agrobacterium strains. J. Mol. Biol. 86:109-127.
