The validation of diagnostic tests is crucial and must consider the end use throughout the process. This includes accounting for the biology and diversity of the pathosystem, the test's intended environments, and its application in a broader diagnostic context. Here, we present the
DAVN framework for assay development and validation, based on
Groth-Helms et al. 2023
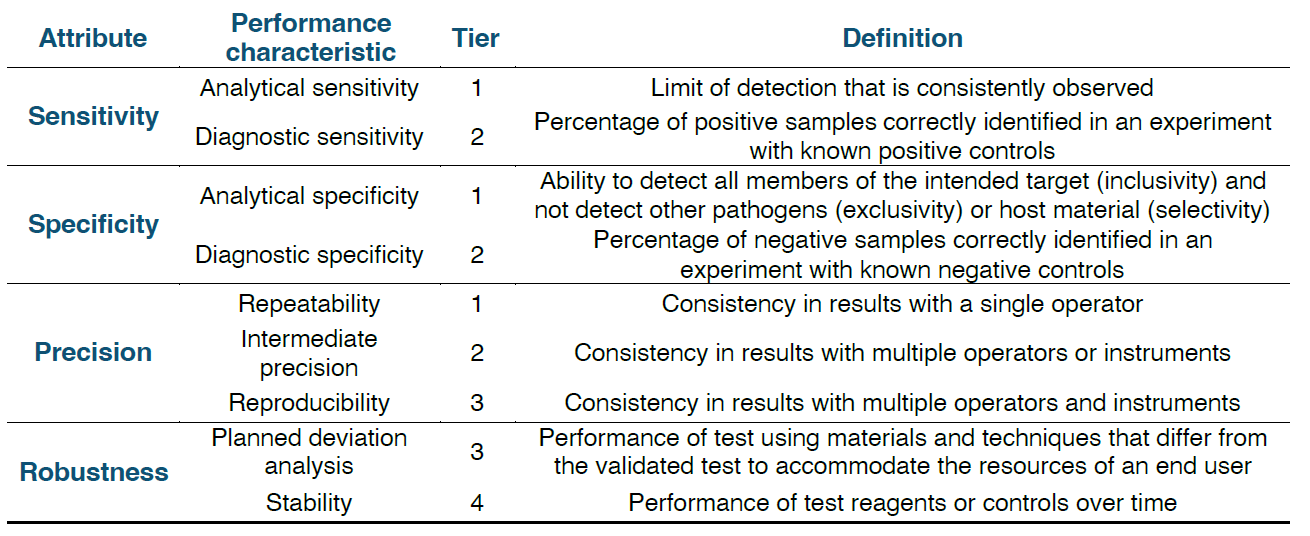
Key attributes such as
specificity, sensitivity, precision, and robustness are consistently assessed, ensuring that positives are reliably identified as positive and negatives as negative for every end user. Validation schemes can vary significantly based on test targets, technologies, and applications, but certain performance characteristics are consistently evaluated regardless of these differences.
The validation framework can be summarized in three main ideas: The
Validation Tier Overview which illustrates the hierarchical tiers for assay validation, the
Workflow of Assay Development and Validation which includes the details of validation workflows, and a table of
Key Terminology that defines each of the key attributes to be assessed by the validation procedure.
Consistent use of validation terminology is essential for developers and stakeholders to understand a test's fitness for a specific purpose. Descriptors of test performance can be found in the Diagnostic Assay Terminology glossary at the end of this page, and authors are encouraged to mention the validation tier level in their publications to facilitate appropriate adoption by end users.
If your assay development follows this framework, please cite Groth-Helms, D., Rivera, Y., Martin, F.N., Arif, M., Sharma, P. and Castlebury, L.A., 2023. Terminology and guidelines for diagnostic assay development and validation: Best practices for molecular tests. PhytoFrontiers™, 3(1), pp.23-35.
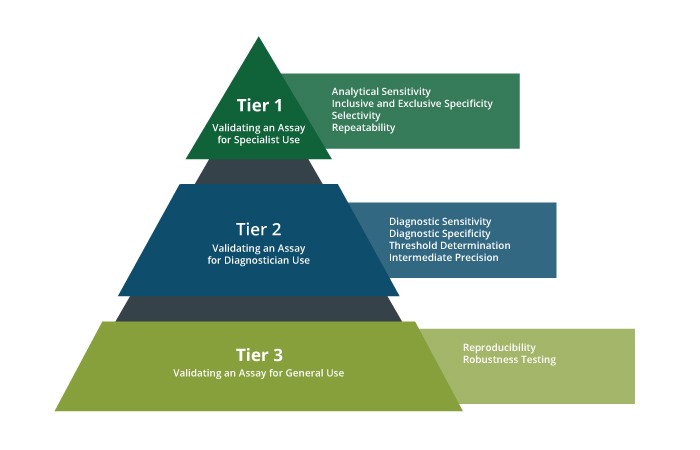
Validation Tier Overview
Validation is tiered as shown in the pyramid below, with Tier 1 focusing on analytical sensitivity and specificity, Tier 2 broadening inclusivity and exclusivity panels for pathogen surveillance, Tier 3 emphasizing reproducibility in various laboratory settings, and Tier 4 evaluating fitness for use in international diagnostics.
Each performance characteristic is part of a larger validation scheme which varies in complexity depending on the intended use of the assay. In general, as a test is used by more people in more places, there are more variables affecting test performance that need to be anticipated and assessed. Below is an overview of how and when these validation descriptors are measured:
Workflow for assay design based on DAVN terminology

Key terms for validation assays and validation tier assignments