Background Information
Instructor Notes
Cast of Characters
Oscar Granados, a field manager, who oversees banana production at a farm belonging to a large multinational fruit company.
Rafael Madriz, a project manager in the fruit company, which exports fruits to demanding international markets in Europe and the U.S.
Daniel Arias, a first-year undergraduate student at the University of Costa Rica, majoring in plant protection, who works as an intern on the farm.
Francisco Vargas, a plant pathology professor at University of Costa Rica.
Mauricio Guzman, a plant pathologist at the National Banana Corporation (CORBANA).
The Case
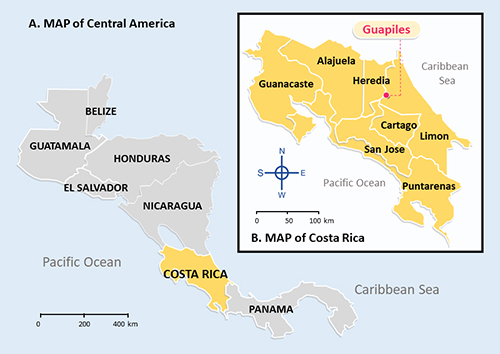
Figure 1 A. Map of Central America showing the location of Costa Rica in Central America, B. Map of Costa Rica showing location of the banana plantation in Guapiles. |
Costa Rica, a nation located in Central America (
Figure 1A), is one of the world's leading exporters of bananas. Oscar Granados is a field manager in charge of all aspects of banana production at a plantation located near the east coast of Costa Rica (
Figure 1B). As part of a multinational fruit-producing company, the plantation exports 5 million pounds of bananas to U.S. and European markets each year. The farm employs about 300 workers year-round, mainly in the field and the packing house.
Sr. Granados received a phone call from Sr. Rafael Madriz, the Costa Rican manager of the multinational fruit company, who complained about the increasing cost of producing bananas on the plantation.
Sr. Madriz: “Hi Oscar, I've checked the latest financial reports of your farm. The expenses for the past year are much too high. Ten years ago, you made only 40 fungicide sprays per year, but now you're putting on 70 sprays per year. Just for comparison, I talked to the managers of banana plantations in Ecuador and Guatemala; they're using only 40 sprays per year, so their costs are lower than yours. Your productivity in boxes of bananas per year is high, but the plantations in those other countries are more profitable, mainly because it's costing you so much to control black Sigatoka. I don't like to say this, but something will have to give. We can't have lower yields or lower fruit quality, but the disease control budget needs to be cut substantially. I need a plan from you to significantly reduce production costs and improve profitability within 6 months, or we will have to abandon the banana plantation."

Figure 2. Severe defoliation of a banana plant (cv. Cavendish) infected with black Sigatoka (Courtesy M. Guzman)
|
Sr. Granados was alarmed by the phone call, but not surprised. He has been the field manager on this plantation for 10 years and has witnessed a steep increase in fungicide sprays over that time. Not only was the cost of fungicide sprays rising, but they were also losing one type of fungicide after another due to the development of resistance by the fungus
P. fijiensis, which causes a disease called black Sigatoka. The disease damages the leaves (Figure 2); when it is severe, it causes premature ripening of the fruit and reduces the market grade of the harvest.
The main strategy for control of black Sigatoka is aerial spraying of fungicides by plane or helicopter (Figure 3). When Sr. Granados first joined the plantation 10 years ago, 40 sprays per year were sufficient to control the disease. By last year, the spraying frequency had increased to 70 sprays, which were applied by a farm management company with which the plantation contracted. At the moment, controlling black Sigatoka accounts for about 30% of the plantation's total production costs.

Figure 3. Aerial spraying of fungicides on a banana plantation to control black Sigatoka. (http://www.musarama.org; Courtesy L. de Lapeyre de Bellaire)
|
While Sr. Granados was struggling with developing the plan requested by Sr. Madriz, he was showing a new summer intern, Daniel Arias, the current situation on the plantation and the formidable disease management problem he was facing.
Daniel just finished his first year in the School of Agronomy at the University of Costa Rica (UCR). During the summer break, he landed an internship at the plantation, which is close to his hometown of Guapiles in eastern Costa Rica. Daniel grew up on a small local farm. His family used to grow and sell their bananas to multinational companies, but their business declined with the spread of various diseases, especially black Sigatoka. Due to worsening disease pressure, the costs of disease control were so high that they could no longer make a profit. As a result, his family shifted several years ago to producing other crops. With his unpleasant memories of the devastation caused by black Sigatoka, Daniel was eager to find out how the disease could be controlled more effectively.

Figure 4. Banana with hard black seeds in the fruit. (Courtesy M. Guzman)
|
Sr. Granados told Daniel the story of the long, bitter battle waged by banana growers against black Sigatoka.
One major challenge in developing a more effective way to manage black Sigatoka relates to the nature of the modern cultivated banana plant itself. Seedless bananas were selected for by early banana farmers, who took advantage of natural mutations that resulted in seedless fruit. Seedless bananas are more pleasant to eat than the ancestral bananas, which had hard black seeds (Figure 4). However, seedless bananas are a genetic dead end, since seeds are needed when crossing parent plants for developing improved banana types.
For the past 60 years or so, the export banana market around the world has been dominated by a cultivar called Cavendish, which is very susceptible to black Sigatoka; that is, they become infected readily and are vulnerable to severe damage. Since modern export bananas are almost always seedless, they can be propagated only by vegetative methods, which means that all Cavendish banana plants in export-producing plantations are genetically identical clones.
Another set of challenges comes from the realities of producing export-quality bananas in eastern Costa Rica. Banana is a perennial crop with continuous production of shoots from the base of older plants, and a plantation can be sustained for more than 10 years. The climate in eastern Costa Rica is warm and rainy all year, which enables year-round banana harvesting but also creates non-stop pressure from the black Sigatoka fungus. Unlike the production of most crops, the banana crop cycle in eastern Costa Rica is never interrupted by winter, or even by a dry season. The climate is just as ideal for black Sigatoka as it is for banana. As a result,
P. fijiensis can build up on diseased parts of banana leaves, producing spores that can spread the disease further, aided by rain and wind. This never-ending cycle of black Sigatoka means that export-quality bananas need protection virtually 365 days per year.
What happens when constant disease pressure meets frequent fungicide sprays? In eastern Costa Rica, the result has been the development of resistance in the black Sigatoka fungus to fungicide after fungicide. Ironically, some of the same fungicides that turned out to be most vulnerable to resistance development were initially among the most potent weapons against the disease.
Knowing the reality of the intensive fungicide application schedule and the health risks of the contact fungicides, Daniel couldn't help worrying about the health of workers on the banana farm and the environmental pollutants that may be damaging the neighborhood where he lives. He hoped to help Sr. Granados come up with a sustainable strategy that can reduce the pesticide load in banana production. He thought about asking advice from Professor Francisco Vargas from University of Costa Rica (UCR), who is an expert in plant pathology, and Mauricio Guzman, who is a plant pathologist at the National Banana Corporation (CORBANA) in Guapiles. Fungicides are grouped by how they act on the plant. “Contact" fungicides land on the plant surface and stay there; they act on fungi only on the outer surface of the plant. “Systemic" fungicides, on the other hand, can enter the plant tissues and stop infections that are already started. The Achilles heel of most systemic fungicides, however, is their vulnerability to being overcome by the development of resistance in the fungus. When systemic fungicides, such as triazoles, were first introduced, the interval between sprays was 21 to 28 days. The interval has become shorter and shorter as the fungus became insensitive to triazoles. This story has played out repeatedly in eastern Costa Rica over the past 40 years, as class after class of systemic fungicides has become ineffective against the black Sigatoka fungus. In earlier years, many of the contact fungicides fell out of use when the systemic fungicides entered the market, but now they have returned in the wake of resistance to the systemics. Unlike systemic fungicides, many contact fungicides have little risk of resistance development, because they act broadly on the metabolic pathways of a fungus rather than narrowly like the systemics. Unfortunately, the environmental and human health risks of older contact fungicides, such as mancozeb and chlorothalonil, are generally higher than for the systemics, and their effectiveness on the plant tends to be shorter-lived since they can be washed off by rain. The disappearance of the systemics due to resistance to black Sigatoka has forced banana plantations to use these older contact fungicides, which must be applied every 5 to 7 days, rather than every 10 to 14 days as for the systemics.
Daniel called Prof. Vargas and asked for better solutions to control black Sigatoka than steadily intensifying fungicide use. Prof. Vargas said that the current fungicide spray program to control black Sigatoka is not a sustainable practice. Ideally, breeders could improve the host resistance to black Sigatoka, but this is very challenging due to the sterility (i.e., lack of seeds) of the Cavendish bananas that are used for export. Even cultivars with seeds are difficult to cross because commercial bananas are genetic triploids, meaning that they have three sets of chromosomes, which don't pair up well during sexual reproduction. Even without these barriers, it usually takes many years to develop new cultivars with desired traits. Daniel knew that adopting disease-resistant banana cultivars is a long-term goal, but he needed a quick plan to help Sr. Granados reduce fungicide applications in 6 months. He called Sr. Guzman at CORBANA for alternative solutions.

Figure 5. Removing infected leaves infected with black Sigatoka using a de-leafing knife. (Courtesy M. Guzman)
|
Sr. Guzman suggested several cultural practices to control black Sigatoka. The warm temperature and high humidity in eastern Costa Rica favor germination and development of
P. fijiensis, as the fungus needs water for growth and spreading its spores around. To slow down the disease, it is helpful to reduce the duration of periods of high humidity in the field. Factors that encourage persistence of high humidity in a banana plantation include slow drainage of rainfall and high plant density. A good time to check if drainage systems need to be improved is after a heavy rain when it is easy to see if water is being removed rapidly. Waterlogging is detrimental to banana growth. Giving plants adequate space can help improve air circulation by allowing leaves to dry quickly after rainfall. Reducing plant density (i.e., the number of banana plants per unit area in the plantation) would also improve spray coverage of fungicides. Besides lowering soil and air moisture, improving soil fertility could help by resulting in healthier leaves that can support high productivity.

Figure 6. Spraying urea (nitrogen fertilizer) on detached banana leaves to accelerate their decay and reduce sources of black Sigatoka spores. (Courtesy M. Guzman)
|
Another possible way that Sr. Guzman suggested to reduce the risk of black Sigatoka epidemics is to remove infected parts of leaves (Figure 5). If more than half of the leaf area shows symptoms of black Sigatoka, it is preferable to remove the entire leaf. De-leafing is labor-intensive, but if done on a regular basis can keep the spore levels low, especially during periods of prolonged rainfall. Knowing the right time to remove the leaves is also vital, as you don’t want to sacrifice too much leaf area that is needed for photosynthesis. Removing infected leaves before applying a systemic fungicide can improve the efficiency of fungicide treatment. Detached leaves can be placed on the ground, where composting them in piles or rows can kill spores and reduce infection risk. Application of urea, a nitrogen fertilizer, to the compost rows has been used to accelerate the process of decomposition (Figure 6).
Sr. Granados was excited to learn about these strategies, but he had some concerns about de-leafing. Should he hire more people and train them to help? It would increase the budget quite a bit if it needed to be done frequently. He wasn’t sure whether it would be worth the extra time and money and the extent to which it could reduce the need for fungicide sprays. Should he stick to the intensive fungicide spray schedule?

Figure 7. A plantation technician scouting for black Sigatoka symptoms. (Courtesy M. Guzman)
|
Daniel Arias called Prof. Vargas again to get more suggestions on ways to reduce the cost of fungicide spraying against black Sigatoka. Daniel knew a little from his plant pathology course at UCR about the application of disease-warning systems for disease control. A warning system can take some of the guesswork out of fungicide spray decisions by tracking the relationship between weather conditions and the development of disease. Daniel asked Prof. Vargas how a warning system could be used in decision-making for timing fungicide sprays to control black Sigatoka. Prof. Vargas explained that relative humidity of the atmosphere is a useful index. The disease risk is high during periods when relative humidity is high (for example, above 90%) and low during drier periods. Any practice that can reduce the number of hours per day that the plantation has humidity above 90% could slow down the disease; the fewer hours above the humidity threshold, the better. Furthermore, assessing the level of the disease is very useful in evaluating the efficiency of the fungicide applications. A simple practice is to keep track of 10 banana plants per acre and record the severity of symptoms weekly (Figure 7). The risk of damage from black Sigatoka can be estimated by using the weather conditions in a given week. If the risk of infection in a particular week is relatively low, a cheaper (slightly less effective) fungicide can be used; but if the risk is high, more expensive and effective fungicides will be needed. Therefore, the company can save some money by using relatively inexpensive fungicides when conditions are less favorable for the disease.
As an alternative to chemical fungicides, the use of the pathogen’s natural microbial competitors to control its populations can also help control fungal disease, which is referred to as biological control. For example, fungi in the genus
Trichoderma have been used to reduce the severity of black Sigatoka. Using biological agents is more environmentally friendly and less harmful to human health than using chemical fungicides. However, the process of commercializing biological control products takes many years. Finding an effective biological control can depend on the identification of native strains adapted to local conditions, as well as developing effective ways to increase colonization of biological agents on banana plants for a prolonged period, so they don’t have to be reapplied frequently.
Acknowledgments
We thank Dr. Leonor Leandro at Iowa State University for providing access to her course, Principles of Mycology (MICRO 456), for the class tryout. We also thank the undergraduates in MICRO 456 class for providing valuable suggestions for improving this case study.
Selected References
Bennett, R. S., & Arneson, P. A. (2003). Black Sigatoka of Bananas and Plantains.
The Plant Health Instructor. https://doi.org/10.1094/PHI-I-2003-0905-01
Cavero, P. A. S., Hanada, R. E., Gasparotto, L., Coelho Neto, R. A., & de Souza, J. T. (2015). Biological Control of Banana Black Sigatoka Disease with Trichoderma.
Ciência Rural,
45(6), 951–957. https://doi.org/10.1590/0103-8478cr20140436
Ganry, J., Foure, E., de Bellaire, L. de L., & Lescot, T. (2012). An Integrated Approach to Control the Black Leaf Streak Disease (BLSD) of Bananas, while Reducing Fungicide Use and Environmental Impact. Fungicides for Plant and Animal Diseases.
InTech. https://doi.org/10.5772/29794
Marín, D. H., Romero, R. A., Guzmán, M., & Sutton, T. B. (2003). Black Sigatoka: An Increasing Threat to Banana Cultivation.
Plant Disease,
87(3), 208–222. https://doi.org/10.1094/PDIS.2003.87.3.208
Ploetz, R. C. (2001). Black Sigatoka of Banana: The Most Important Disease of a Most Important Fruit.
The Plant Health Instructor. https://doi.org/10.1094/PHI-I-2001-0126-02
Saville, A., Charles, M., Chavan, S., Muñoz, M., Gómez-Alpizar, L., & Ristaino, J. B. (2017). Population Structure of
Pseudocercospora fijiensis in Costa Rica Reveals Shared Haplotype Diversity with Southeast Asian Populations.
Phytopathology,
107(12), 1541–1548. https://doi.org/10.1094/PHYTO-02-17-0045-R
