As with many inventions, "development" of the first fungicide was the result of good observations. The first use of brining of grain with salt water followed by liming took place in the middle of the 17th century to control bunt, and followed the observation that seed wheat salvaged from the sea was free of bunt. This had occurred long before Tillet (1755) established that seed-borne fungi (Tilletia tritici,
T. laevis) caused bunt of wheat and that it could be controlled by seed treatments of lime, or lime and salt. Another important discovery was made in France in 1882 by Millardet, who noticed that grape vines that had been sprayed with a bluish-white mixture of copper sulfate and lime to deter pilferers retained their leaves through the season, whereas the unsprayed vines lost their leaves. After numerous spraying experiments Millardet concluded that a mixture of copper sulfate and hydrated lime could effectively control downy mildew of grape. Hooker in 1923 stated in his paper on colloidal copper hydroxide that there were no entirely satisfactory fungicides available. Then he went on to describe the shortcomings of the Bordeaux mixture and lime-sulfur, the latter also being "most disagreeable to handle." Up until the 1940s chemical disease control relied upon inorganic chemical preparations, frequently prepared by the user.
Many of the early efforts to produce healthy crops involved diseases that had newly been introduced and left the growers quite helpless. In the following sections we highlight trends in fungicide development and use over the last century in light of the ever-changing spectra and intensities of fungal pathogens, which have often occurred as a consequence of changing cropping systems.
Development of the Importance of Diseases
Since Tillet presented the results of well replicated and controlled experiments in 1755, in which he added black dust from bunted wheat to seed from healthy wheat and observed that bunt was much more prevalent in plants produced from such seed than from non-dusted seed, researchers and growers have been combating plant pathogens in various ways. The importance of epidemics was highlighted by late blight of potato in Europe in the 1840s which led to several severe famines. Key among the researchers at this time was DeBary, whose studies on the Peronosporaceae and the discovery of alternate hosts in the life cycle of rust fungi were two of his many important contributions. At that time it took 100 years for man’s knowledge to double, today it is done in 16 years. In the following 150 years much has been learnt about the control of plant diseases and several complementary approaches were developed for their control (Table 1). Depending on the crop, the disease and the availability of control methods, a different set of approaches is employed.
Table 1. Key methods of controlling plant diseases.
| Regulatory measures | Quarantine, inspection and seed certification |
| Cultural methods | Crop rotation, sanitation, improved growing conditions |
| Biological methods | Breeding of resistant varieties, microbials |
| Chemical control | Seed treatment; soil, foliar & post harvest applications |
When looking at disease losses, the most devastating ones occur post harvest, as these include the entire costs incurred in producing the crop. One of the greatest discoveries in plant pathology in the twentieth century was that of Flor in the 1940s when he proposed his gene for gene theory, based on results of his crosses of flax, each of which were resistant to none, one, or several races of flax rust (3). Flor found that both resistance in plants as well as avirulence in the pathogen were inherited, and that both traits were dominant. Plant breeders at universities and seed companies have invested thousands of man-years in breeding crops resistant to diseases since that time. It may take as long to breed a new resistance gene into a new cultivar as to introduce a new fungicide (± 10 years), even though this time can be shortened somewhat today with molecular tools by reducing the back-crossing cycles. Pathogens may overcome both means of control by the selection of adapted strains in a short period of time, thereby invoking the old adage that
nature abhors a vacuum.
More recently, the trend towards more intensive cropping, the introduction of high yielding varieties and the neglect of proper crop rotation have led to new diseases in some crops. Minimum tillage or direct seeding without any plowing as well as mechanization of harvesting in fruit trees or grapes can also change the spectrum of diseases for which the growers need to find control measures. Such changes in crop varieties and in production methods keep the disease picture very dynamic so that new methods for disease control are constantly needed.
Overview of Fungicide Development and Usage
Up to 1940. Through the study of diseases that caused clear economic damage and the study of epidemiology of the pathogens, the basic principles of disease control were established. The major products used up to 1940 are listed in Table 2. In general, chemical disease control was aimed at horticultural crops (fruit and vegetables) as well as seed treatments. Concerns for the products’ impact on the environment were largely non-existent, as were concerns for the applicator. Most users prepared their own fungicides from basic recipes.
Table 2. Fungicides in use up until 1940 [after Russell 2005 (15)]
|
Year |
Fungicide |
Primary Use |
| 1637 | Brine | Cereal seed treatment |
| 1755 | Arsenic | Cereal seed treatment |
| 1760 | Copper sulfate | Cereal seed treatment |
| 1824 | Sulfur (dust) | Powdery mildew and other pathogens |
| 1833 | Lime sulfur | Broad spectrum foliar pathogens |
| 1885 | Bordeaux mixture | Broad spectrum foliar pathogens |
| 1891 | Mercury chloride | Turf fungicide |
| 1900 | CuOCl2 | Especially
Phytophthora infestans |
| 1914 | Phenylmercury chloride | Cereal seed treatment |
| 1932 | Cu2O | Seed and broad spectrum foliar diseases |
| 1934 | Dithiocarbamates patented | Broad spectrum protectants |
| 1940 | Chloranil, Dichlone | Broad spectrum seed treatment |
Proprietary products were available at this time for those who did not want to take the time and trouble to make their own. Prior to the introduction of the dithiocarbamates, the testing of which was described by McCallan of Cornell University in 1930, most of the products used as fungicides were applied at high rates, e.g., 10 to 20 kg a.i./ha (~9 to 18 lbs/acre) for sulfur against powdery mildew on grapes. The products did not always give good control, could be phytotoxic and had to be applied frequently. Fig. 1 shows the reduction in the tonnage of fungicide used in the U.S. between 1944 and 2002. This is a reflection of the drastic reduction of use rates per ha (acre) as more effective and selective fungicides were introduced over this time. For example, the current use rates of well below 100 gr/ha (1.4 oz/acre) for many triazoles against the same pathogen is a 200 fold reduction.
| |
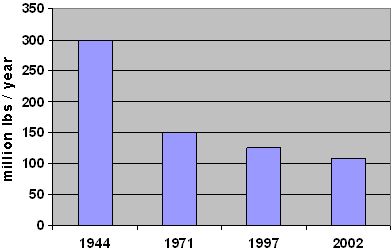
Fig. 1. U.S. Crop Protection Fungicide Use (5). | |
1940 to 1970. From 1940 to 1970 there were a number of new chemistry classes introduced as fungicides (Table 3). The dithiocarbamates and later the phthalimides represented a major improvement over the previously used inorganic fungicides in that they were more active, less phytotoxic and easier to prepare by the user. The fungicide research climate of that time is well described by Horsfall (7), who was instrumental in the discovery and the introduction of the dithiocarbamates, the most widely used group of organic fungicides.
Table 3. Key classes of fungicides introduced between 1940 and 1970.
|
Fungicide class |
Active Ingredient and Year |
| Dithiocarbamate | thiram 1942, zineb, nabam 1943, maneb 1955, mancozeb 1961 |
| Aromatic Hydrocarbon | biphenyl 1944 |
| Phthalimide | captan, folpet 1952, captafol 1962 |
| Fentin | fentin acetate, fentin hydroxide 1954 |
| Antibiotic | blasticidin S 1955, kasugamycin, polyoxin 1965 |
| Triazine | anilazine 1955 |
| Guanidine | dodine 1957 |
| Nitroanaline | dicloran 1960 |
| Benzimidazoles | thiabendazole 1964, benomyl 1968, thiophanate methyl 1970 |
| Phthalonitrile | chlorothalonil 1964 |
| Morpholine | dodemorph 1965, tridemorph 1969 |
| Carboxanilide | Carboxin 1966, oxycarboxin 1966 |
It is worth noting that during this time period: (i) several of these classes of chemistry also gave rise to herbicides — e.g., triazines and nitroanalines, (ii) an antibiotic was introduced to control rice blast in Japan and is classified as a systemic and, (iii) the swing had taken place to the reliance on commercially produced products helping to give rise to the crop protection industry. With this swing came the interest in the biochemical mode of action of fungicides. Most of these fungicides were being used at 1.5 to 3 kg a.i./ha (~1.3 to 2.7 lb/acre) (see Fig. 1).
The decade from 1960 to 1970 saw a rapid expansion of research and development along with a rapid growth of the fungicide markets. In this decade, the most widely used protectant fungicides, mancozeb and chlorothalonil, were introduced. The decade also gave us the first broad-spectrum foliar systemic, thiabendazole, and the systemic seed treatment carboxin. Much of this new chemistry arose as a result of the basic manufacturers having moved away from
in vitro screens to
in vivo screens. In these
in vivo screens, young potted plants were sprayed or drench treated with the test compounds and inoculated with a major pathogen soon thereafter. The plants then were placed in a greenhouse or growth chamber under ideal disease conditions until disease symptoms appeared and they could be rated for contact or systemic efficacy as well as for phytotoxicity. This process was usually repeated on a weekly basis with large numbers of test compounds.
Beyond 1970. The more important modern fungicides introduced since 1970 are listed in Table 4 according to their mode of action or chemical class. For additional information on mode of action and resistance risk, see also Table 5. A discussion of the key classes of modern fungicides follows.
• Benzimidazole fungicides were introduced for plant disease control in the 1960s and early 1970s as foliar fungicides, seed treatments and for use in post harvest applications. They possessed unique properties not seen before in the protectants. These included low use rates, broad spectrum and systemicity with post-infection action that allowed for extended spray interval. All these qualities made them very popular with growers but also subject to misuse, such as poor spray coverage and curative spraying. The first case of resistance to benzimidazoles occurred in powdery mildew in greenhouses in 1969, one year after introduction. By 1984, resistance had been reported on many of the pathogens against which benzimidazoles are active (17). The reason for the rapid development of resistance was that these fungicides were single site inhibitors of fungal microtubule assembly during mitosis, via tubulin-benzimidazole-interactions. The primary patent holders of this class were DuPont (Benlate), Merck,Sharp & Dohme (Mertec) and Nippon Soda (Topsin M). The current ranking of global sales is: carbendazim, thiophanate, thiabendazole.
• Morpholine fungicides are best known for their excellent control of cereal diseases, powdery mildew on vegetables and grapes, and sigatoka of banana. During the 1980s fenpropidin and fenpropimorph were key fungicides in the European cereal market, while tridemorph was used extensively for sigatoka. This class of chemistry, although having seen shifts in sensitivity by some pathogens (sigatoka in Central and South America), is still in use. Key patents were held by BASF (Calixin and Corbel) and Dr. R. Maag (Corbel and Tern). Dimethomorph, though a morpholine, is quite distinct from the morpholines above with its activity against Oomycetes via the inhibition of cell wall formation (FRAC group 40 in Table 5). The current ranking of global sales is: dimethomorph, fenpropidin, fnpropimorph, sprioxamine.
Morpholine fungicides belong to a broad group of fungicides that is often referred to as sterol biosynthesis inhibitors (SBI). Other SBIs include the next four groups of fungicides (see also Table 5).
• Piperazines: the major player in this group was triforine, which was used extensively as a home and garden product (especially on roses). Key to the acceptance of triforine was its efficacy and safety to a wide range of plants. The key producer was CelaMerck (Saprol).
• Imidazoles include a small number of compounds in this class that are active against plant pathogens. The most important are imazalil (Janssen) and prochloraz (Boots). The primary uses for imazalil were as a seed treatment and post harvest treatment, while prochloraz (Sportak) was used on cereals, being especially active on
Pseudocercosporella eyespot.
• Pyrimidines are a class of fungicides that were extensively explored by Eli Lilly giving rise to nuarimol, fenarimol and triarimol. The major player of these was fenarimol (Rubigan) on pome fruit, grapes and turf.
• Triazoles are the largest class of fungicides (see Fig. 2). Bayer was the first to launch a triazole, namely triadimefon (Bayleton) in 1973. This was soon followed by triadimenol (Baytan) and bitertanol (Baycor). Janssen Pharmaceuticals sold the agricultural use rights to Ciba-Geigy for propiconazole (Tilt) which was launched in 1979. Numerous other triazoles have been launched since, with Bayer’s most recent entrée being prothiaconazole (Proline) in 2004. The reason for the longevity of this class of fungicides is that while being highly efficient broad spectrum products, resistance has occurred over time as a slow shift resulting in a decreased sensitivity to their mode of action as de-methylation inhibitors (DMI). The newer triazoles, being intrinsically more active, push the sensitivity curves back to their original ED 50 values. The current ranking of global sales is: tebuconazole, epoxiconazole, propiconazole, difenoconazole, flusilazole, tetraconazole, fluquinconazole, flutriafol.
• Anilides are a diverse group of fungicides. The earliest introduction was anilazine (Dyrene), primarily as a leaf spot fungicide from Bayer and Nissan, followed by the seed treatment carboxin (Vitavax), which is highly effective on bunts, smuts and assorted Basidiomycetes such as
Rhizoctonia spp. This was followed by the dicarboximides iprodione (Rovral) from Rhone-Poulenc, vinclozolin (Ronilan) from BASF and procymidone (Sumisclex) from Sumitomo. These fungicides all had exceptional protectant activity on the genera
Botrytis, Monilinia and
Sclerotinia. Combating resistance became an issue with the wide scale use of these fungicides.
Unquestionably the greatest of this group of anilides were the phenylamide fungicides metalaxyl (Apron/ Ridomil) from Ciba–Geigy and benalaxyl (Galben) from Isagro. These, along with phosphonate fosetyl-Al (Aliette) from Rhone-Poulenc, which was also introduced in 1977, brought a completely new level of control to the Oomycetes through their systemic properties by offering protection to the plants as seed treatments, and soil or foliar applications. Oxadixyl (Sandofan) from Sandoz was a later member of the phenylamides. Recently, Syngenta (1996) with mefenoxam (Apron XL and Ridomil Gold) and Isagro (2005) with kiralaxyl have introduced the resolved isomers of metalaxyl and benalaxyl. Again, what has limited the use of the phenylamide fungicides has been the development of resistance, even though the manufacturers tried introducing combinations with protectant fungicides such as mancozeb and chlorothalonil.
The latest anilide to be registered (2003) is boscalid (Emerald, Endura, Pristine) from BASF. Boscalid is registered for foliar use on a wide range of vegetables, fruits and nut crops, either alone or in a mixture with pyraclostrobin as Pristine.
• Strobilurins, launched in 1996, are now the second largest chemistry group of fungicides (Fig. 2) as a result of widespread use on cereals and, more recently, on soybeans (a market that reached $600 million in 2004). Companies have recently also promoted the plant health attributes of this group of fungicides on soybeans and corn. The strobilurin fungicides have a broad spectrum, are highly efficacious, and are suitable for a wide range of crops. Some problems with disease resistance are affecting sales (e.g.,
Septoria in wheat in Europe, and the U.S. turf market). As a result, companies are adjusting the use recommendations by developing mixtures and other uses, including seed treatments.
Table 4. Major fungicide groups introduced since 1970 with their most important representatives.
|
Group |
Year |
Common name of compounds |
Main spectrum /
uses |
Inhibitors
of sterol biosynthesis (triazoles
if not indicated otherwise) | 1973 | triadimefon
imazalil (imidazole) | broad
post harvest & seed |
| 1975 | fenarimol (pyrimidine) | powdery mildew |
| 1977 | triadimenol
prochloraz (imidazole) | seed treatment
cereal fungicide |
| 1979 | propiconazole, bitertanol
fenpropimorph (morpholine) | broad
broad / cereals |
| 1982 | triflumizole | broad |
| 1983 | flutriafol, diniconazole, fluzilazole,
penconazole |
broad |
| 1986 | fenpropidin (morpholine)
hexaconazole, cyproconazole,
myclobutanil,
pyrifenox (pyridine)
tebuconazole | broad / cereals
broad
broad / leaf crops
broad, foliar & seed |
| 1988 | difenoconazole tetraconazole,
fenbuconazole | broad, foliar & seed broad |
| 1990 | epoxiconazole | broad / cereals |
| 1992 | metconazole, fluquinconazole
triticonazole | broad
broad, foliar & seed |
| 2002 | prothioconazole | broad |
Inhibitors of cytochrome bc1
(Qo site &
strobilurin analogues
if not indicated otherwise) | 1992 | azoxystrobin
kresoxim-methyl | broad
cereal fungicide |
| 1996 | famoxadone (azolone) | oomycetes |
| 1998 | fenamidone (azolone)
trifloxystrobin | oomycetes
broad |
| 2000 | picoxstrobin
pyraclostrobin, fluoxastrobin | cereal fungicide
broad |
| 2001 | cyazofamid (Qi site of action, cyanoimidazole) |
Oomycetes |
|
Other classes, various fungicides and plant activators |
Common names with
year of introduction |
Main
spectrum
/
uses |
| Dicarboximides | iprodione 1974, vinclozolin 1975, procymidione 1976 | Botrytis, Monilinia |
| Phenylamides | metalaxyl 1977, benalaxyl 1981, oxadixyl 1983, mefenoxam 1996 | Oomycetes |
| Phenylpyrroles | fenpiclonil 1990, fludioxonil 1990 | broad foliar and seed |
| Anilinopyrimidies | pyrimethanil 1992, cyprodinil 1994 | broad |
| Melanin synthesis | tricyclazole 1975, pyroquilone 1985, carpropamide 1997 | rice / water and foliar |
| CAA fungidices* | dimethomorph 1988, iprovalicarb 1998, benthiavalicarb 2003, mandipropamid 2005 | Oomycetes |
| Defense activators | probenazole 1979, acibenzolar-S.methyl 1996 | fungi, bacteria, viruses |
| Various | cymoxanil 1976, fosetyl-Al 1977, propamocarb 1978, | Oomycetes |
| carbendazim 1976, fluazinam 1992 | broad |
| quinoxyfen 1997 | powdery mildew |
*
carboxylic acid amides
Zeneca began researching this chemistry in the early 1980s, first synthesizing azoxystrobin (Amistar, Abound, Quadris) in 1988; it is now the largest selling member of this group. However, kresoxim-methyl (Cygnus, Sovran) from BASF was the first member to be commercialized in 1996. BASF has since entered the market (2002) with a broader spectrum strobilurin pyraclostrobin (Cabrio, Headline, Insignia) also sold in combination with kresoxim-methyl as Opera and with boscalid as Pristine. Other strobilurin fungicides include, trifloxystrobin (Flint) discovered by MAAG but sold by Bayer, and fluoxastrobin (Disarm, Evito) discovered by Bayer but sold by Arysta. The current ranking of global sales is: azoxystrobin, pyraclostrobin, trifloxystrobin, kresoxim-methyl, picoxystrobin.
• Other Systemic Fungicides include a diverse group of products, such as: tricyclazole (Beam) launched in 1975 by Eli Lily/Dow and still widely used for control of rice blast; cymoxanil (Curzate), a downy mildewcide from DuPont; the cereal and fruit fungicide cyprodinil (Vanguard, Unix) from Syngenta; fludioxonil (Saphire, Switch, Maxim) from Syngenta; and quinoxyfen (Fortress, Quintec), a powdery mildewcide from Dow.
Newer active ingredients introduced recently are benthiavalicarb (Valbon from Kumiai) and mandipropamid (Revus from Syngenta), from the carboxylic acid amide (CAA) fungicide group, and fluopicolide (Infinito from Bayer), metrafenone (Flexity from BASF), proquinazid (Talius from DuPont), and zoxamide (Electis from Dow). For a more detailed technical description of modern fungicides, including chemistry, we refer to the treatise edited by Krämer and Schirmer (10).
One of the most novel new products introduced by Ciba-Geigy is acibenzolar-S-methyl (Actigard, Bion). At use rates of 30 gr/ha (~0.4 oz/acre) or less, it activates the host’s systemic acquired resistance (SAR) process in many crop plants. It offers broad protection against fungi, bacteria and viruses without having any direct activity on these pathogens (11). Actigard has performed best when incorporated into a program of chemical sprays, as the inherent level of disease control has seldom been sufficient when applied alone. This product has initiated a whole new field of research into utilizing peptides for controlling diseases, and other means of stimulating SAR and the Jasmonic acid pathway (JA) with chemicals and biological agents in plants. Probenazole has been used successfully against rice blast since 1979 and was later shown to activate defense mechanisms in rice.
Fungicide Resistance Management
In Fig. 2 market shares for the major chemical groups of fungicides are summarized for 2005 including non-crop uses. According to this source, the youngest group, the stobilurines, had surpassed within a few years all of the older fungicides groups in importance except only the DMIs. This indicates that the innovation potential has been substantial for fungicides with new modes of action and excellent performance, not least because some of the older groups have suffered from an erosion of efficacy against some important pathogens due to the emergence of resistance [see also FRAC 2007 (1)]. The non-crop uses include amenity grass, which is the single largest fungicide market in the U.S. The numerous pathogens that attack these highly maintained grasses, such as those found on golf courses, frequently require weekly spray applications through out the summer. This has already led to resistance development in
Magnaporthe grisea (gray leaf spot) to strobilurins.
| |

Fig. 2. Percent market share of major chemical classes of fungicides for 2005 (including non-crop use) (14). | |
Thus, a major consideration for the design of fungicide use strategies is the threat of fungicide resistance. There have been considerable efforts by industry to conduct research in the areas of mode of action, resistance risk, field monitoring for baseline sensitivity and sensitivity variations in treated fields. Based on the results, use strategies are designed that reduce the risk of fungicide resistance build-up or worse, the loss of efficacy of whole fungicide classes.
This threat of fungicide resistance and the fact that cross-resistance often exists to related products from different manufacturers has lead to a close collaboration between them in FRAC (Fungicide Resistance Action Committee). Results from research in mode of action, resistance risk and field monitoring are pooled and strategies are developed to minimize the risk of resistance build-up. FRAC has produced several monographs on various aspects of fungicide resistance and has grouped the available fungicides according to various criteria that facilitate the understanding of the resistance risk of the different fungicide groups (Table 5). Such assessments of the resistance risk are made difficult by the unpredictability of cross-resistance, which, in most cases, is clear-cut and follows the mode of action but in other cases can be quite complex. In the end, what matters for practical purposes, is not so much the mode of action of a fungicide but its cross-resistance to other fungicides.
Table 5. Mode of action of major fungicides classes, their FRAC code and resistance risk. For additional information, see the
FRAC Code List.
|
FRAC Code |
Chemical Class |
Mode of action / inhibition |
Resistance risk |
| 1 | Benzimidazoles | Beta-tubulin biosynthesis | high |
| 2 | Dicarboximides | NADH cytochrome c reductase in lipids | high |
| 3 | Azoles, Pyrimidines | C-14 demethylation in sterol biosynthesis | medium |
| 4 | Phenylamides | RNA polymerase | high |
| 5 | Morpholines | ^8 and ^7 isomerase and ^14 reductase in sterol biosynthesis | low-medium |
| 7 | Carboxamides | Succinic acid oxidation | medium |
| 9 | Anilinopyrimidine | Methionine biosynthesis | medium |
| 11 | Strobilurins | Mitochondrial synthesis in cytochrome bc1 | high |
| 16 | Various chemistry | Melanin biosynthesis (two sites) | medium |
| 40 | Carboxylic acid amides | Cell wall formation in Oomycetes | low-medium |
| M1 | Inorganics | Multisite contact | low |
| M3 | Dithiocarbamates | Multisite contact | low |
| M5 | Phthalimides | Multisite contact | low |
The Benefits and Risks of Fungicides
The application of any chemical to a crop or food raises the question of risks and benefits. This discussion of risk has shifted from dealing with the toxicity to the user in the field and the consumer (4) to a much wider focus that includes the whole environment and the ecosystem in which the crops are growing (16). As a consequence more and more studies are required before a fungicide can be used, leading to enormous development costs. This leads industry to concentrate on the big markets, while smaller markets are increasingly left out and in urgent need of effective fungicides. In the U.S. the IR-4 program has been established to provide safe and effective pest management solutions for specialty crop growers. In December 2007 the UN-FAO held a global minor use summit along with IR-4 and EPA to establish global residue zones and standard data requirements.
Overall, most analyses come to the conclusion that the benefits of fungicides far outweigh the risks, if they are used carefully and according to the label recommendations. Currently more than 80% of the fruit and vegetable crops grown in the U.S. receive a fungicide every season. The benefit of fungicide use in the U.S. agriculture is said by Gianessi and Reigner (5) to boost farm income by nearly $13 billion annually. The alternatives proposed by organic farmers, who are opposed to intensive farming altogether, exclude the use of synthetic fungicides, but allow the use of copper and sulfur based inorganic fungicides. There is still an ongoing debate as to whether traditionally or organically grown products are safer for the consumer. For example, a growing number of studies are being conducted to evaluate the risk of mycotoxins in the two farming systems.
The Future of Fungicides
A look at the history of fungicides should give us some idea of what to expect in the future. The major changes in fungicide use have usually been associated with changes in the spectra of pathogens as well as in crop intensities, practices or prices. The migration of tobacco blue mold into Europe or soybean rust into the Americas had a dramatic impact on the fungicide use on these crops. Fig. 3 shows the value of fungicides of the major crop groups and illustrates the importance of the dynamic nature of the fungicide market. Cereals, especially the foliar applications, represent a relatively recent market when compared to fruits and vegetables. The soybean market was negligible until a few years ago when rust reached the large soybean acreages in North and South America. Such shifts in pathogen spectra could not be foreseen and will continue to occur in the future due to increased global trade with plant material. Similarly the dramatic increase in cropping intensity in European cereal production in the 1960s and 70s created a major market where practically none existed before. The remarkable ability of new pathogens to adapt to intensively cultivated cereals has led to a large list of pathogens that can threaten these crops. More generally, a growing world population that wants to be fed better than today will lead to increased areas of intensive cropping and hence, most likely, fungicide use will also increase. The higher demand on some grain commodities for use as biofuel has already resulted in very low carryover stocks of grain, which will inevitably result in price increases (2). This exerts pressure for further intensification of these crops. Corn in the U.S. is a good candidate for fungicide sprays becoming a regular production practice.
| |

Fig. 3. Global fungicides market share for the major crop groups for 2005 (14). | |
The trend towards a more judicious use of fungicides in conjunction with disease forecast systems that has been observed in the past can be expected to continue in the future. This will help reduce the risk of adaptation by the target fungi and at the same time will reduce residues in the environment and on the produce. The efforts of breeding for disease resistance will also continue and possibly increase by utilizing the tools of genetic engineering. Both genetic resistance and selective fungicides are prone to adaptation by the pathogen. The balance between genetic and chemical control will therefore most likely continue and research in both areas will complement each other to assure the availability of effective combinations of host resistance and fungicides for crops that should produce ever higher yields of ever better quality.
Currently the biocontrol products contribute less than 1% of the fungicide market; undoubtedly they will contribute more in the future, with the most likely avenue being through natural compounds known as biopesticides. An interesting new area of research is the use of antimicrobial peptides (AMP) for improving resistance to pathogens using transgenic plants as bio-factories for fungicides or bactericides.
Thus the major multi-national companies will be focusing their future on an integration of genetic traits and agrochemicals. Projected growth of the agrochemical market is 3.4% over the next 5 years, whereas traits are forecast to grow at 7%. The average current (2005) R & D expenditures of the six major Agro companies is 64.5% for chemistry and 35.5% for seed and traits. This ensures that new fungicides will continue to be developed to protect the ever more precious cultivars, where they do not have sufficient genetic disease resistance. This balance between genetic resistance and disease control products of chemical, biochemical or biological nature will remain and is not likely to change dramatically in the near future. Equally important for sustainable disease control will be the intelligent integration of these technologies with sound cultural and sanitation measures.
Literature Cited
1.
Brent, K. J., and Hollomon, D. W. 2007. Fungicide Resistance in Crop Pathogens: How Can It Be Managed? 2nd Rev. Edn. Online. Fungicide Resistance Action Committee (FRAC). CropLife Int'l., Brussels, Belgium.
2. Collins, J. C. 2007. Challenges and opportunities in crop production over the next decade. Pages 3-12 in: Pesticide Chemistry, Crop Protection, Public Health, Environmental Safety. H. Ohkawa, H. Miyagawa, and P. W. Lee, eds. Wiley VCH Verlag, Weinheim, Germany.
3. Flor, H. H. 1955. Host parasite interaction in flax rust – its genetics and other implications. Phytopathology 45:680-685.
4. Frazer, A. C. 1963. Balance of pesticides: Benefits and risks. Pages 3-11 in: Proc. 2nd British Crop Protection Conference..
5. Gianessi, L., and Reigner, N. 2006. The importance of fungicides in U.S. crop production. Outlook on Pest Management 10:209-213.
6. Hooker, H. D. 1923. Colloidial copper hydroxide as a fungicide. Indust. Engin. Chem. 15:1177-1178.
7. Horsfall, J. G. 1975. Fungi and fungicides: The story of a nonconformist. Ann. Rev. Phytopathol. 13:1-14.
8. Jones, L. R. 1914. Problems and Progress in Plant Pathology. Am. J. Bot. 1:97-111.
9. Kelman, A., and Peterson, P. D. 2002. Contributions of plant scientists to the development of the germ theory of disease. Microbes Infect. 4:257-260.
10. Krämer, W., and Schirmer, U., eds. 2007. Modern Crop Protection Compounds, Vol. 2. Wiley-VCH Verlag, Weinheim, Germany.
11. Leadbeater, A., and Staub, T. 2007. Exploitation of induced resistance: A commercial perspective. Pages 229-242 in: Induced Resistance for Plant Defence. D. Walter, A. Newton, and G. Lyon, eds. Blackwell, Oxford, UK.
12. McCallan, S. E. A. 1930. Studies on Fungicides II. Testing protective fungicides in the laboratory. Cornell Agric. Exp. Stn. Memoirs 128:8-24.
13. McCallan, S. E. A. 1967. History of fungicides. Pages 1-37 in: Fungicides, An Advanced Treatise, Vol. I. Academic Press, New York, NY.
14. Phillips McDougall. 2006. Phillips McDougall Agriservice Report. Pathhead, Midlothian, Scotland, UK
15. Russell, P. E. 2005. A century of fungicide evolution. Journal of Agricultral Science 143:11-25.
16. Schlundt, H. 2002. Risks and benefits of biological and chemical plant protection strategies – food safety aspects. Proc. British Crop Protection Conference 2002, pp. 3-21.
17. Smith, C. M. 1988. History of benzimidazole use and resistance. Pages 23-24 in: Fungicide Resistance in North America. C. J. Delp, ed. American Phytopathological Society, St. Paul, MN.
