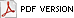
Phakopsora pachyrhizi, the causal agent of Asian Soybean Rust (SBR) (Fig. 1), has been documented on many legume hosts (3,20,23,25,30). However, it is best known for its devastating effect on soybean yields in Asia and South America (2,6,13), and has received much media attention in the United States. In November 2004, the disease was first detected in the continental United States in a production soybean field on a research farm near Baton Rouge, Louisiana (6,26). In the following weeks, the disease was found in Alabama, Arkansas, Georgia, Florida, Mississippi, Missouri, South Carolina and Tennessee (6,22). At that time, many researchers felt that the disease could become widespread in the Southeast and Midwestern states in 2005. However, by year’s end, SBR was only observed in the Southeast. The disease did not spread to the Midwestern soybean producing states, perhaps due to low inoculum levels, low spore viability, and/or poor environmental conditions for SBR development.
| |

Fig. 1. Symptoms of Asian Soybean Rust (Phakopsora pachyrhizi) (SBR) on lower soybean leaf surface. Photo by L. Sconyers. |
|
This article presents a brief history of SBR, and a description of symptoms and disease development. The results of sentinel plot monitoring in Georgia will be presented, as well as a Southeastern perspective on SBR development in 2005. We will conclude with a discussion on fungicide trial results, disease management, and an outlook for 2006.
History
Soybean rust is caused by the obligate parasitic fungi Phakopsora pachyrhizi and Phakopsora meibomiae. Asian soybean rust, the disease responsible for significant yield losses in Asia and South America, is caused by P. pachyrhizi, which is the more aggressive of the two pathogens. P. meibomiae has been documented in the Caribbean Basin, including Puerto Rico, but has not caused any known severe yield losses in soybean (20). Asian soybean rust is spread by urediniospores carried by wind and wind-blown rain, and can potentially cause significant economic damage in soybean-producing areas. The development of the disease is dependent on sufficient inoculum and conducive environmental conditions. A potential source for inoculum includes over-wintering kudzu (Pueraria lobata) which may be found in the Southeastern United States, Caribbean Basin, Central America, and South America (6,15,16,19).
SBR was first discovered in Japan in 1902. From there, the disease spread to other areas of Asia, Australia, and then to India and Africa. Kenya, Rwanda, and Uganda reported SBR in 1996; Zambia and Zimbabwe reported SBR in 1998. Nigeria, Mozambique, and South Africa reported SBR in 1999, 2000, and 2001, respectively (1,3,6,7,12,14,27). Asian soybean rust was reported in Hawaii in 1994 (9). Wind currents carried spores across the Atlantic Ocean to Paraguay in 2001, Brazil and Argentina in 2002, and Bolivia and Colombia in 2003 (6,20,21,24). The disease rapidly reached devastating levels in South America, where yield losses have ranged from 10 to 80% in some production fields (20,21,24,29).
On 6 November 2004, symptoms consistent with SBR were discovered in the continental United States on a 5-acre production soybean field on a research farm near Baton Rouge, Louisiana and were confirmed to be SBR on 10 November by the USDA-APHIS laboratory in Beltsville, Maryland (6,26). In the weeks that followed, SBR was confirmed in Alabama, Arkansas, Georgia, Florida, Mississippi, Missouri, South Carolina, and Tennessee (6,22).
Based on epidemics in South America from 2001 to 2004, many in the scientific community felt that the disease would quickly develop in North America in 2005. Potential yield losses for the United States crop in 2005 were estimated to be between 10 and 50%, but as much as 80% if no action was taken for disease management (6,13,20). The development of the disease to this level would require optimal environmental conditions, and an over-wintering source of the pathogen. It was suspected that the disease would survive on kudzu (Pueraria lobata) found in southern no-frost regions in the United States, or be blown into North America from the Caribbean Basin, Central America, or South America. However, SBR levels did not reach those predicted for 2005.
Symptoms and Disease Development
Phakopsora pachyrhizi produces urediniospores (serving as primary and secondary inoculum) that are carried by wind or windblown rain. Like many rusts, SBR has the potential to move and develop very quickly. Spores land on the leaf surface, produce germ tubes, then penetrate the epidermis directly (Fig. 2A). Subsequent growth results in hyphae and the development of haustoria (10). Volcano-shaped pustules emerge from the abaxial leaf surface approximately 10 to 14 days after infection (Fig. 2B). These structures can produce urediniospores for at least three weeks (6,11,15).

A |
|

B |
Fig. 2A & 2B. Asian Soybean Rust urediniospores at 400× (A),
and volcano-shaped pustules at 60× on kudzu (B).
Photo (A) by J. Brock and photo (B) by L. Sconyers.
Symptoms of SBR first appear as tiny tan to red lesions on the adaxial leaf surface (Fig. 3). On soybeans, symptoms typically occur first on older leaves located in the lower portions of the canopy. On kudzu, symptoms seem to be more prevalent in partially shaded areas. These lesions can be very small and easily overlooked by the observer. Spore masses may be seen with a 20× or greater hand lens; however, young infections (one to two clear to tan pustules per leaf) may go undetected. A dissection microscope (40 to 60×) is most effective for detection of young SBR infections. Symptoms of other soybean diseases, such as brown spot (Septoria glycines), bacterial pustule (Xanthomonas axonopodis pv. glycines), and downy mildew (Peronospora manshurica), are often confused with SBR, thus complicating disease detection.
| |

Fig. 3. Brown-red lesions caused by Phakopsora pachyrhizi on the upper soybean leaf surface. Photo courtesy of USDA. |
|
Rapid disease development has been correlated with canopy closure and bloom stage (R1+) (6,11,18). Asian soybean rust progresses until complete defoliation of the crop, or until the environment is no longer conducive for disease development. Severely infected soybeans have fewer, smaller, and lighter weight seeds which result in reduced yields.
Asian soybean rust can develop quickly provided that the environmental conditions are favorable. Frequent rain events, long dew periods and temperatures ranging from 15°C to 29°C appear to be optimal for SBR development (6,16,19). However, in 2005 soybean leaves with sporulating pustules were detected in the Southeast on days in which temperatures were in excess of 32°C.
2005 Spread of Asian Soybean Rust
The spread of Asian soybean rust across the Southeastern United States occurred much more slowly than expected, given prevailing environmental conditions immediately following the first detection. It is possible that hot temperatures and dry weather may have reduced infection efficiency and slowed the rate of the epidemic. However, hot temperatures did not eliminate disease spread. According to the chronology of positive detections for 2005 on the United States Department of Agriculture Soybean Rust Information website, P. pachyrhizi was detected first on kudzu in February in Pasco County, Florida. The fungus was later detected on volunteer soybeans in April in Seminole County, Georgia. For nearly two months, the fungus was not detected, although weather conditions associated with multiple tropical storms seemed favorable for disease development. The next find was not until 14 June 2005 when the fungus was found on roadside kudzu in Jefferson County, Florida.
Further spread of the disease was slow from June to July, despite seemingly optimal SBR conditions. During this time, many soybean cultivars planted in the Southeast were approaching the bloom stage (R1). June was typified by cooler than average temperatures (23°C to 25°C on average), and widespread rainfall events in the Southeast (www.noaa.gov).
SBR detections finally began to increase in August when soybeans were reaching R3-R4 growth stages. Positive detections continued through November. Overall, 35, 10, 47, and 22 counties in the United States reported SBR in August, September, October and November, respectively. This increase in the number of detections occurred during a time in which temperatures rose by 5-10 degrees on average and rainfall decreased (www.noaa.gov). With a few exceptions, SBR was not detected in many commercial soybean fields until the R4 stage or later (R. Kemerait and P. Jost, personal communication). In Georgia, the first commercial soybean field detection occurred 18 August in Appling County when soybeans were at the R4 growth stage.
Not only was SBR slow in establishment in 2005, but disease development was not always in the uniform gradient often associated with airborne plant diseases. In many areas of the Southeast (Florida, Alabama, Georgia and South Carolina), it was noted that SBR began in discrete focal points in the lower soybean canopy within a field. The disease would then move upward within the soybean canopy and to adjacent soybean plants within approximately 7-10 days, spreading sporadically over the entire field. These discrete focal points suggest that either inoculum levels were low at the time of spore deposition, or that spores were deposited in aggregates.
While P. pachyrhizi has been documented on many legumes (3,20,23,25,30), kudzu has been the only host documented as an over-wintering source for the fungus in North America. Although the fungus was detected on kudzu in many areas of the Southeast, there were many kudzu patches that did not develop disease. This may have been due in part to differences among kudzu accessions. Research conducted by Sun et al. suggests that multiple introductions of kudzu from many sources, followed by subsequent gene exchange and recombination, may result in kudzu having different responses to pathogens or biocontrol agents (28). Thus the sporadic and patchy detection of P. pachyrhizi on kudzu in the Southeast may have been due to different kudzu populations having different levels of susceptibility or resistance to the fungus. Another potential explanation for the sporadic occurrence in kudzu is that spores were deposited in foci as described previously.
In November 2005, spores and pustules consistent with P. pachyrhizi were observed on Florida Beggarweed (Desmodium tortuosum) growing in Attapulgus, Georgia, near a heavily infected soybean trial (Fig. 4a and 4b). Subsequent PCR analyses were conducted on infected D. tortuosum in Tifton, Georgia and Beltsville, Maryland and were positive for the fungus. To our knowledge, this is the first report of P. pachyrhizi on D. tortuosum. The importance of this find and its implication for over-wintering of this rust fungus is still being determined.

A |
|

B |
Fig. 4A and 4B. Detection of Phakopsora pachyrhizi pustules at 60× (A) and spores at 200× (B)
on a Florida Beggarweed sample (Desmodium tortuosum) from Attapulgus, Georgia in 2005.
Photo A by L. Sconyers and Photo B by J. Brock.
Sentinel Monitoring in Georgia and the Southeast
The progress of Asian soybean rust development was monitored in Georgia (as with all other participating states, see Table 1) as part of the USDA-APHIS and the North Central Soybean Research Program (NCSRP) SBR scouting program. In 2005, 25 sentinel plots were established in Georgia in order to provide early detection to soybean producers. Data from these plots were used to initiate fungicide spraying for a particular location and to provide epidemiological data for predictive models (Fig. 5). The Georgia sentinel system included 17 soybean plots, 5 roadside kudzu patches, and 3 clover (Trifolium sp.) patches. These plots were examined weekly for SBR development by randomly collecting 100 leaves and examining them both with a hand lens and a dissection microscope. Soybean planting (predominately determinate varieties) in Georgia typically occurs from May 10 to June 10, with some varieties planted as late as June 30 (8). The 17 soybean sentinel plots, consisting of maturity group II, III and IV soybeans, were planted from the first to third week of April on research stations and commercial farms. Data recorded from sentinel plots included Global Positioning System coordinates, planting date, cultivar name, row spacing, planting density, plant height, vegetative and reproductive growth stages, degree of canopy closure, SBR incidence (number of leaves infected of 100 collected), and severity (low, moderate or heavy). Of the 25 Georgia sentinel plots established in 2005, SBR was detected in 1 kudzu and 13 soybean sentinel plots. Asian soybean rust was not detected in clover. By 13 November, SBR had been confirmed in 34 counties (Fig. 5).
Table 1. Minimumz distribution of sentinel plots in 2005.
| State |
USDA |
NCSRPy |
| Alabama |
10 |
20 |
| Arkan | sas |
15 |
20 |
| Delaware |
5 |
|
| Florida |
15 |
20 |
| Georgia |
10 |
20 |
| Illinois |
10 |
20 |
| Indiana |
10 |
20 |
| Iowa |
10 |
20 |
| Kansas |
10 |
20 |
| Kentucky |
10 |
20 |
| Louisiana |
15 |
20 |
| Maryland |
5 |
|
| Michigan |
10 |
20 |
| Minnesota |
10 |
20 |
| Mississippi |
15 |
20 |
| Missouri |
10 |
20 |
| Nebraska |
10 |
20 |
| New Jersey |
5 |
|
| New York |
5 |
|
| North Carolina |
10 |
|
| North Dakota |
10 |
20 |
| Ohio |
10 |
20 |
| Oklahoma |
5 |
|
| Pennsylvania |
5 |
|
| South Carolina |
10 |
|
| South Dakota |
10 |
20 |
| Tennessee |
15 |
20 |
| Texas |
10 |
|
| Virginia |
5 |
|
| West Virginia |
5 |
|
| Wisconsin |
10 |
20 |
| Colorado |
5 |
|
| Idaho |
5 |
|
| Oregon |
5 |
|
| Washington |
5 |
|
| Grand Total |
315 |
|
z Minimum number of plots proposed to have been established
in 2005 based on funding available for sentinel monitoring.
Some states established more plots than the minimum
requirement.
y North Central Soybean Research Program
|

Fig. 5. Distribution of soybean rust positive counties (in red) and soybean rust negative counties (in green) in Georgia as of 3 November 2005. Numbered asterisks correspond to clover, kudzu or soybean sentinel plots. |
|
In Georgia, the first positive detection of SBR occurred in Seminole County in April on volunteer soybeans, and the first SBR detected in a sentinel plot occurred in Tift County on 15 July. The last find of SBR on a sentinel plot was in Oconee County, Georgia on 24 August. Based on the elapsed time and distance from the Tift County and Oconee County SBR detections, it was estimated that disease in Georgia was moving north at approximately 60 miles per week.
Florida’s first report of the SBR pathogen in 2005 was on kudzu in Feburary in Pasco County, and by 13 November, 23 Florida counties reported SBR. The first report of SBR in Alabama in 2005 occurred in June on soybean in Baldwin County, and the first commercial field identified with SBR in the United States in 2005 was in Baldwin County, Alabama on 12 July (E. Sikora, personal communication). By 13 November, there were 31 counties positive with SBR in that state. Mississippi had 2 counties reporting SBR. The first find in South Carolina occurred in soybean in August in Hampton County, and by 13 November, 23 counties had been reported positive for SBR in South Carolina. SBR was not detected in North Carolina until October, with the first find in Brunswick County on late season soybeans. By 13 November, 15 counties reported SBR in North Carolina. Liberty County, Texas was the only county in that state with confirmed P. pachyrhizi, and it was detected in early November on kudzu that was believed to have been killed by frost the first week of December 2005 (T. Isakeit, personal communication). Despite having SBR confirmed first in 2004, Louisiana was the last state in 2005 to detect SBR. One parish was positive for SBR in Louisiana as of 13 November. A complete list of counties, dates, and hosts with positive detection of the SBR pathogen is available online.
Asian soybean rust was widespread by the end of the growing season in the Southeast; however, northern spread in the region appeared to be slow. As of 13 November 2005, when most of the United States crop had been harvested, the northern most find of SBR was Edgecombe County, North Carolina. The eastern most find was Hyde County, North Carolina. Liberty County, Texas is the farthest west that SBR has been detected (Fig. 6) (USDA Soybean Rust Information website).
| |

Fig. 6. Distribution of Asian soybean rust in the United States as of 13 November 2005. Photo available at www.sbrusa.net. |
|
Spore Trap Monitoring
As part of a study conducted by Syngenta and the University of Arkansas, spore traps provided by Syngenta (Fig. 7) were placed in the center of 10 soybean sentinel plots in Georgia (# 6, 7, 8, 10, 16 17, 18, 22, 23, and 25 in Fig. 5). The traps were used to collect spores onto a microscope slide coated with petroleum jelly. The slide was placed inside of a plastic tube that was used to capture wind-blown spores. The purpose of these traps was to provide an additional warning for SBR by detecting the presence of P. pachyrhizi-like urediospores that may lead to subsequent disease development. Slides for these traps were replaced weekly and sent to the University of Arkansas for analysis and spore enumeration.
| |

Fig. 7. Collecting soybean leaf samples, and a Syngenta-provided spore trap placed in the center of a Washington County, Georgia sentinel soybean sentinel plot in 2005. Photo by L. Sconyers. |
|
Spore traps that were placed in the 10 soybean sentinel plots in Georgia also provided information concerning the correlation of P. pachyrhizi-like spores trapped and disease detection (Table 2). Asian soybean rust was detected 5 to 55 days after P. pachyrhizi-like spores were trapped in 5 counties. However, P. pachyrhizi-like spores were trapped 28 June in the Appling County sentinel, but disease was never detected in that plot. For one site, Tift County (trap 1), P. pachyrhizi-like spores were not trapped until 31 days after disease was detected. Despite the presence of P. pachyrhizi-like spores, disease never occurred in the sentinel plots for Tift County (trap 2), Toombs County, and Burke County. This illustrates that the presence of spores does not necessarily lead to SBR development, or not all spores were P. pachyrhizi. Spore viability, concentration, and environmental conditions are also critical for disease development.
Table 2. Difference in time (days) between Phakopsora pachyrhizi-like spores trapped and disease detection in ten sentinel plot locations in Georgia in 2005.
| County |
Rust-like spores
trapped |
Disease
detected |
Difference
in days |
| Colquitt |
5 July |
3 August |
+31 |
| Laurens |
29 July |
3 August |
+5 |
| Washington |
5 July |
30 August |
+55 |
| Sumter |
5 July |
18 August |
+34 |
| Decatur |
28 June |
21 July |
+23 |
| Tift (trap 1)z |
15 August |
15 July |
-31 |
| Tift (trap 2)z |
21 July |
None |
NA |
| Appling |
28 June |
None |
NA |
| Toombs |
None |
None |
NA |
| Burke |
None |
None |
NA |
z Two sentinel plots were located in Tift County (trap 1: Lang Research Farm; trap 2: RDC Field).
Teliospores of P. pachyrhizi have been reported in Florida on kudzu; however, this stage in the epidemiology and spread of SBR in the United States is still not fully understood (P. Harmon, personal communication).
The significance of finding ‘rust-like’ spores was of some debate across the United States. Some state specialists chose not to release that information for fear of initiating premature spray applications when disease was non-existent, leading to unnecessary production costs to the grower. Other states chose to release spore find information for fear that they would be accused of withholding information from the public. Another concern is that there are many different plant rust species that might be confused with P. pachyrhizi; the presence of these spores could signal a false alert. With further study and some modifications, these spore traps may become more reliable in the detection of SBR spores. Fluorescent proteins for tagging of actual SBR spores or improved polymerase chain reaction (PCR) assays for the detection of low numbers of SBR spores could improve this particular tool for state specialists (J. Rupe, personal communication).
Management of Asian Soybean Rust
Historically, foliar fungicide applications on soybean have not been a common practice in the United States, except for managing late-season diseases in the Mississippi River basin (29). Properly applied, efficacious foliar fungicides will now be needed for the management of SBR. To be effective, fungicides must be applied deeply into the canopy in order to reach initial infections. In larger fields, while aerial applications may offer convenience and do not damage foliage as do tractor-mounted spray systems (Fig. 8), they may not provide the same degree of canopy penetration as a ground application. Efficacy of fungicide treatments also depend on proper calibration of spray equipment, soybean growth stage and environmental conditions (6). In Georgia in 2005, effective applications were achieved with flat-fan nozzles, utilizing high spray pressure and fine to medium spray droplets (6).
| |

Fig. 8. Boom-mounted (top photo) and aerial (bottom photo) fungicide applications in Terrell, County, Georgia. Photo by W. Duffie. |
|
In 2005, eight replicated trials were conducted in Georgia to evaluate fungicides and to determine optimal application timings for managing SBR (Fig. 9). Strobilurins, triazoles, benzimidazoles, chlorothalonil, and various other products were evaluated. In these experiments, soybeans were planted and grown in a manner consistent with Extension recommendations from the University of Georgia (8). Plots were planted from May 10 to June 10 with Roundup Ready® soybeans of maturity group VII (determinate soybeans). Fungicide applications were initiated when disease was found in the fungicide plots or when disease was detected in local sentinel plots. For most trials, the initial application occurred at first bloom (R1), and the second application occurred 3 weeks later at the beginning of pod formation (R3). Applications were made with either a backpack sprayer or tractor mounted boom equipped with flat-fan nozzles calibrated to deliver 20 gallons of water per acre at 50 psi or greater. Data collected included SBR incidence (determined from 20 terminal leaflets collected randomly from mid to low canopy), severity (percentage of each leaf infected: 0 = no SBR, 1 = less than 5%, 2 = 5-15%, 3 = 15-35%, 4 = 35-67.5%, 5 = greater than 67.5%), estimated percentage of canopy defoliation due to SBR, and yield (bu/acre).
| |

Fig. 9. 2005 fungicide trial locations in Georgia. Compiled by R. Kemerait. |
|
Asian soybean rust development varied among trial locations. One trial in Attapulgus, Georgia had a uniform development and treatment differences were clear to assess (Fig. 10). In some locations, such as the Lang Research Farm in Tifton, Georgia, disease development was not uniform throughout the trial. In this trial, disease was initially detected at corner of the field, and slowly spread across the trial resulting in a gradient of disease severity (Fig. 11). As a result, clear differences among treatments were difficult to assess due to the variation in the SBR development in that trial.
| |
 |
|
 |
|
| |
Fig. 10. Aerial photograph of a fungicide trial in Attapulgus, GA. Photo was taken as natural defoliation was beginning to occur and illustrates effect of spraying fungicides for managing SBR. Plots with efficacious fungicide treatments have green leaves, while the less effective applications and unsprayed plots are brown and defoliated. Photo by A. Mathis. |
|
Fig. 11. Aerial photograph of a fungicide trial at the Lang Research Farm in Tifton, GA. Notice the variation in SBR defoliation throughout the field. Spore deposition and subsequent SBR infections appeared to have begun in the bottom left corner of the field. Photo by A. Mathis. |
|
In trials where pyraclostrobin (Headline) was applied, there was a noted greening effect in which leaves remained greener longer and defoliation was delayed (Fig. 10). This occurred to a lesser degree with azoxystrobin (Quadris). Delayed defoliation did not affect percent moisture content at harvest, but did result in greater amounts of foreign matter in the yields.
Some phytotoxicity was observed in commercial fields and fungicide trials where tebuconazole (Folicur) or tebuconazole plus pyraclostrobin (Headline SBR) was applied. The same symptoms were noted in plots treated with the experimental fungicide metconazole in a trial in Attapulgus, Georgia. This phytotoxcity appeared as interveinal chlorosis (Fig. 12), similar to soybean sudden death syndrome and nematode infestation; however, no yield loss was noted as a result.
| |

Fig. 12. Interveinal chlorosis and necrosis which resembles sudden death syndrome and nematode damage resulting from applications of tebuconazole. Similar symptoms have also been noted on soybeans sprayed with metaconazole. Photo by R. Kemerait. |
After one year of trials, it is difficult to determine the "best" product for management of SBR. Data from a trial conducted in Macon County, Georgia suggests that Folicur, Headline, Headline SBR, Laredo, and Stratego performed well in managing SBR in Georgia in 2005 (Table 3). It was observed that the disease could be managed with strobilurins, triazoles, and strobilurin plus triazole tank mixtures. Severe levels of disease in unsprayed plots did not increase the severity of disease in adjacent sprayed plots; therefore, growers should not be overly concerned about neighbors not spraying their fields. Further data concerning the efficacy of various products for the management of SBR will be published by the various states that conducted trials in 2005 in the 2006 Fungicide and Nematicide Tests.
Table 3. 2005 fungicide trial results for Macon County, Georgia. Two applications were applied beginning at first bloom (26 August) (R1) and 3 weeks later (9 September) (R3). Similar lower case letters indicate no significant difference among treatments for leaflets infected or among treatments for severity. (Fisher’s Least Significant Difference t-test; P = 0.05).
| Treatment rate (per acre) |
No. leaflets
infectedz |
Average severity
per leaflety |
| Untreated |
20.0 a |
5.0 a |
| Folicur (tebuconazole), 4.0 oz |
16.7 ab |
1.2 cd |
Stratego (trifloxystrobin and
propiconazole), 8.0 oz |
19.3 a |
1.0 cd |
| Headline (pyraclostrobin), 9.0 oz |
16.7 ab |
0.9 cd |
| Echo 720 (chlorothalonil), 24.0 oz |
20.0 a |
2.4 b |
| Laredo (myclobutanil), 6.0 oz |
20.0 a |
1.1 cd |
Headline SBR (pyraclostrobin and
tebuconazole), 7.8 oz |
15.3 b |
0.8 d |
| Quadris (azoxystrobin), 10.8 oz |
18.7 ab |
1.7 bc |
z Asian soybean rust infection was assessed by collecting 20 terminal leaflets from the lower canopy of each plot and determining the number of leaflets infected out of 20.
y Based on a visual estimation of percentage of each leaflet infected and rated (20 October) on a scale of 0 to 5, where 0 = no disease, 1 = trace to 5% infection, 2 = 5 to 15%, 3 = 15 to 35%, 4 = 35 to 67.5%, 5 = 67.5 to 100%.
Fungicide application timing was also of interest. It has been documented in South America that the reproductive stages of soybean are the most susceptible to SBR infection, and that applications during vegetative growth stages are not recommended (6,20,21,24). When inoculum was present, first bloom to beginning pod formation (the R1 to R2 stages) was determined to be critical for disease development in the Southeastern United States in 2005. This is consistent with data from South America. In a trial conducted in Appling County, Georgia, disease severity was decreased and yields were increased when fungicides were applied at the R2 growth stage several weeks prior to extensive disease development (Table 4). A delay in fungicide application or no fungicide application not only resulted in an increase in disease, but also a decrease in yield. Additional studies are necessary to examine products and application timings under varying levels and timings of infection and environmental conditions. Since additional fungicides exist for use on soybean elsewhere in the world, these products will also need to be evaluated.
Table 4. 2005 fungicide trial results for Appling County, Georgia. Similar lower case letters indicate no significant difference among treatments for number of leaflets infected, severity and yield. (Fisher’s Least Significant Difference t-test; P = 0.05).
Treatment, rate,
application timingz |
No. of
leaflets
infectedy |
Average
severity
per leafletx |
Yield
(bu/acre) |
| Untreated |
20.0 a |
4.7 a |
50.5 d |
| Headline SBRw, 7.8 oz, R2 and R4 |
0.0 d |
0.0 f |
74.6 a |
| Headline SBR, 7.8 oz, R2 |
5.0 c |
0.4 ef |
69.5 ab |
| Headline SBR, 7.8 oz, R5 |
15.5 b |
0.9 de |
64.3de |
z Only four treatments from the entire trial are presented to illustrate the effect of application timing on disease development and yield.
y Asian soybean rust infection was assessed by collecting 20 terminal leaflets from the lower canopy of each plot and determining the number of leaflets infected out of 20.
x Based on a visual estimation of percentage of each leaflet infected and rated (30 September) on a scale of 0 to 5, where 0 = no disease, 1 = trace to 5% infection, 2 = 5 to 15%, 3 = 15 to 35%, 4 = 35 to 67.5%, 5 = 67.5 to 100%.
w Headline SBR consists of pyraclostrobin and tebuconazole.
As with any disease, there is a concern of fungicide resistance developing in the pathogen, particularly with the use of specific-site mode of action fungicides, such as the strobilurins and triazoles. Resistance to the strobilurins has already occurred in other pathogens in North America (17,31). Combinations and rotations of various fungicide chemistries will need further study regarding efficacy and prevention of fungicide resistance.
Soybean cultivars that provide some resistance to SBR would provide growers with an additional tool in managing this disease. Currently, germplasm studies are being conducted in the United States and world wide to find resistance to SBR. Resistance genes to SBR have been identified; however, the resistance was overcome (4,5,20). Some lines and cultivars with potential resistance to SBR have been identified in a trial currently underway in Attapulgus, Georgia (D. Phillips and R. Boerma, personal communication). Studies will continue here and elsewhere to find sources of resistance that may be incorporated into other soybean varieties suitable for production in the United States.
2006 Outlook
The 2005 soybean growing season has provided researchers, growers and industry representatives with valuable information for 2006, yet there is still a great deal of information needed to understand SBR development and management. Issues that need to be addressed include: over-wintering of the pathogen on kudzu and other possible alternative hosts, spore thresholds required to initiate disease, soybean and kudzu microclimate influences on the epidemiology of the disease, temporal and spatial development of SBR, and additional fungicide and germplasm studies.
Though SBR developed slowly in the Southeast in 2005, the disease has the potential to be more damaging in 2006, as the number of over-wintering urediospores on kudzu in Florida (and other frost-free areas) increase. It is difficult to determine whether SBR will have a significant impact on soybean production in the Midwest, since those areas have winter temperatures that are too cold for the fungus to over-winter. For SBR to develop in those areas, spores must be blown in from over-wintering sites in the Southeastern United States, Central America, South America or the Caribbean Basin. It is possible that SBR could be similar to other corn and wheat rust diseases, in that it may only become a problem in years when winds carry sufficient and viable inoculum north, and environmental conditions are conducive for disease development. In 2005, environmental conditions appeared to be conducive for disease development since there were numerous hurricanes and tropical storms, but perhaps the concentration or viability of spores was not great enough for spread and disease development in the Midwest.
With the information that has been collected to date, and the continued cooperation among state, federal, and private agencies observed in 2005, a tremendous amount of work can be accomplished in 2006. Proactive monitoring of sentinel plots and commercial fields will continue next year. The absence of SBR in major soybean production areas of the Midwest and upper Midwest in 2005 does not mean that the disease will remain confined to the Southeast in 2006. In order to apply fungicides in a timely manner and to effectively manage this disease, early detection is critical. Initial SBR infections can easily go undetected. Often only 1 to 2 pustules were found on soybean or kudzu leaves after examination with a dissection microscope. This level of infection would have been missed by scouting only in the field and would have delayed the initiation of a fungicide program. In some cases, leaves with suspicious lesions needed to be incubated for 24-48 h in a humidity chamber to determine if SBR was present in a particular sample. With the knowledge gained from this year and next, we will begin to build forecast models, warning systems, and provide management programs tailored for the producer in each soybean-producing region in the United States.
Acknowledgments
We thank the USDA, the United Soybean Board and the NCSRP for the funding of sentinel monitoring. The authors would like to thank Mr. Jeremy Kichler and Mr. James Clark, the University of Georgia Extension, and Mr. Billy Wayne Sellers, soybean producer in Appling County, Georgia, for their cooperation in fungicide trials. We thank Dr. Roger Boerma for his cooperation in the Attapulgus, Georgia germplasm trial, and Dr. John Rupe and his staff at University of Arkansas for their spore slide analyses. We also thank all of our colleagues and staff at Auburn University, Clemson University, University of Florida, and University of Georgia for their assistance with this work.
Additional Resources
Pest Alert: Soybean Rust
from PPQ-APHIS-USDA
Diagnostic Methods for Soybean Rust Identification
from PPQ-APHIS-USDA
2005 National Soybean Rust Symposium Proceedings
from Plant Management Network
Soybean Rust
from Plant Management Network
Soybean Rust Information Site
from USDA
Literature Cited
1. Akinsanmi, O. A., Ladipo, J. L., and Oyekan, P. O. 2001. First report of soybean rust (Phakopsora pachyrhizi) in Nigeria. Plant Dis. 85:97.
2. Bromfield, K. R. 1980. Soybean rust: some considerations relevant to threat analysis. Prot. Ecol. 2:251-257.
3. Bromfield, K. R. 1984. Soybean rust, Monograph No. 11. American Phytopathological Society. St. Paul, MN.
4. Bromfield, K. R., and Hartwig, E. E. 1980. Resistance to soybean rust and mode of inheritance. Crop Sci. 20:254-255.
5. Bromfield, K. R., Melching, J. S., and Kingsolver, C. H. 1980. Virulence and aggressiveness of Phakopsora pachyrhizi isolates causing soybean rust. Phytopathology 70:17-21.
6. Dorrance, A. E., Draper, M. A., and Hershman, D. E., eds. 2005. Using Foliar Fungicides to Manage Soybean Rust. NC-504 Land Grant Universities Cooperating. Bulletin SR-2005.
7. Javaid, I., and Ashraf, M. 1978. Some observations on soybean diseases in Zambia and occurrence of Pyrenochaeta-Glycines on certain varieties. Plant Dis. Rep. 62:46-47.
8. Jost, P., Harris, G., Harrison, K., Kemerait, R., McPherson, R., Prostko, E., Roberts, P., Raymer, P., Shumaker, G., and Sumner, P. 2005 Georgia Soybean Production Guide. University of Georgia Cooperative Extension Bulletin CSS-05-02.
9. Killgore, E., and Heu, R. 1994. First report of soybean rust in Hawaii. Plant Dis. 78:1216.
10. Koch, E., and Hoppe, H. H. 1988. Development of infection structures by the direct-penetrating soybean rust fungus (Phakopsora pachyrhizi Syd.) on artificial membranes. J. Phytopathol. 122:232-244.
11. Koch, E., Ebrahim Nesbat, F., and Hoppe, H. H. 1983. Light and electron microscopic studies on the development of soybean rust (Phakopsora pachyrhizi Syd.) in susceptible soybean leaves. Phytopathol. Z. 106:302-320.
12. Kochman, J. K. 1977. Soybean rust in Australia. Pp. 44-48 in: Rust of Soybean--The problem and research needs. R. E. Ford and J. B. Sinclair, eds. International Agricultural Publications, Manila, The Philippines.
13. Kuchler, F., Duffy, M., Shrum, R. D., and Dowler, W. M. 1984. Potential economic consequences of the entry of an exotic fungal pest: the case of soybean rust. Phytopathology 74:916-920.
14. Levy, C., Techagwa, J. S., and Tattersfield, J. R. 2002. The status of soybean rust in Zimbabwe and South Africa. Paper read at Brazilian Soybean Congress, at Fozdo Iguacu, Prarana, Brazil.
15. Marchetti, M. A., Uecker, F. A., and Bromfield, K. R. 1975. Uredial development of Phakopsora pachyrhizi in soybeans. Phytopathology 65:822-823.
16. Marchetti, M. A., Melching, J. S., and Bromfield, K. R. 1976. The effects of temperature and dew period on germination and infection by uredospores of Phakopsora pachyrhizi. Phytopathology 66:461-463.
17. McGrath, M. T., and Shishkoff, N. 2003. First report of the cucurbit powdery mildew fungus (Podosphaera xanthii) resistant to strobilurin fungicides in the United States. Plant Dis. 87:1007.
18. McLean, R. J. 1979. Histological studies of resistance to soybean rust, Phakopsora pachyrhizi Syd. Aust. J. Agric. Res. 30:77-84.
19. Melching, J. S., Dowler, W. M., Koogle, D. L., and Royer, M. H. 1989. Effects of duration, frequency, and temperature of leaf wetness periods on soybean rust. Plant Dis. 73:117-122.
20. Miles, M. R., Fredrick, R. D., and Hartman, G. L. 2003. Soybean rust: Is the U.S. soybean crop at risk? Online. APSnet Feature, American Phytopathological Society.
21. Morel, W., and Yorinori, J. T. 2002. Situacion de la roja de la soja en el Paraguay. Bol de Diulgacion No. 44. Ministerio de Agricultura y Granaderia, Centro Regional de Investigacion Agricola, Capitan Miranda, Paraguay.
22. Mullen, J. M., Sikora, E. J., McKemy, J. M., Palm, M. E., Levy, L. and DeVries-Paterson, R. 2006. First report of Asian soybean rust caused by Phakopsora pachyrhizi on soybean in Alabama. Plant Dis. 90:112.
23. Ono, Y., Buritica, P., and Hennen, J. F. 1992. Delimitation of Phakopsora, Physopella and Cerotelium and their species on Leguminosae. Mycol. Res. 96:825-850.
24. Rossi, R. L. 2003. First report of Phakospora pachrhizi, the causal organism of soybean rust in the Provence of Misiones, Argentina. Plant Dis. 87:102.
25. Rytter, J. L., Dowler, W. M., and Bromfield, K. R. 1984. Additional alternative hosts of Phakopsora pachyrhizi, causal agent of soybean rust. Plant Dis. 68:818-819.
26. Schneider, R. W., Hollier, C. A., and Whitam, H. K. 2005. First report of soybean rust caused by Phakopsora pachyrhizi in the continental United States. Plant Dis. 89:774.
27. Sharma, N. D., and Mehta, S. K. 1996. Soybean rust in Madhya Pradesh. Acta Botanica Indica 24:115-116.
28. Sun, J. H., Li, Z. C., Jewett, D. K., Britton, K. O., Ye, W. H., and Ge, X. J. 2005. Genetic diversity of Pueraria lobata (kudzu) and closely related taxa as revealed by inter-simple sequence repeat analysis. Weed Res. 45:255–260.
29. Sweets, L. E., Wrather, J. A., and Wright, S. 2004. Soybean Rust. University of Missouri-Columbia Cooperative Extension Bulletin G4442.
30. Vakili, N. G. 1979. Field survey of endemic leguminous hosts of Phakopsora pachyrhizi in Puerto Rico. Plant Dis. Rept. 63:931-935.
31. Vincelli, P., and Dixon, E. 2002. Resistance to Q(o)I (strobilurin-like) fungicides in isolates of Pyricularia grisea from perennial ryegrass. Plant Dis. 86:235-240.
32. Yang, X. B. 1995. Assessment and management of the risk of soybean rust. Proceedings of the soybean rust workshop, 9-11 August 1995. J. B. Sinclair and G. L. Hartman, eds. National Soybean Research Laboratory, Urbana, IL.
