Introduction
For the past century, plant virology and the American Phytopathological Society have a deeply intertwined history. As the Society emerged as a distinct entity in the first decade of the 20th century, viruses were also making their mark as newly described and discovered agents of disease. Interestingly, Tobacco mosaic virus (TMV) and its economic hosts in the Solanaceae, such as tobacco and tomato, also find their origins in the Americas.
What follows is a brief review of the origins of our understanding of “the nature of the virus,” deciphering the basis of host-pathogen interaction, and vignettes of the early TMV workers who developed many of the tools and techniques that have become part of the definition of what it is “to be” a virologist or “to do” virology. As plant molecular virology has its origins in the early 20th century, first from the early descriptive work of viruses diseases (1900-1935), followed by the biochemical, the genetics, and biophysical work (1935-1960), the molecular biology (1960-1980), and our current era of transgenic technology, functional genetics of plant viruses, and using viruses as molecular tools, it is useful to develop a contextual understanding of how we came to work with TMV.
The Mosaic Disease of Tobacco
Origins and uses of the genus Nicotiana. The natural distribution of tobacco, genus Nicotiana, is primarily limited to the Americas and Australia. The species that have been cultivated and used for commercial tobacco production originated in South America, particularly the Andean highlands (8). Tobacco was used by pre-Columbian cultures for pleasure, the medicinal narcotic effect, and as a hallucinogen alone or mixed with other plants (6). Two species of tobacco are of particular importance in the historiography of TMV: Nicotiana glutinosa and Nicotiana tabacum. The center of origin for N. glutinosa is north-central Peru and for N. tabacum is northwest Argentina. N. tabacum (tobacco) is distinguished by "its ability to survive in a great variety of situations ... indicating its considerable ecological tolerance" (8) and usefulness in selection of cultivated varieties.
Tobacco cultivation: Setting the stage for disease. In Colonial America, tobacco was a successful cash crop and was used as legal tender (22). However tobacco, as all other successful crops, is often subject to continuous cultivation as monocultures (planting large tracts of a single crop, often times year-after-year in the same plot of land). As a result of intensive cultivation, tobacco depletes the soil of essential nutrients and the plants become more susceptible to disease. One such example is the mosaic disease of tobacco. This disease was observed in intensively cultivated tobacco fields in the Ambalema district of Colombia in the early 19th century. The disease was called amulatamiento, the etymology of which was suggested to be from "el tabaco se ha mulatto" (10). From my translation, this can be interpreted as "the tobacco leaf appeared as mulatto or a mixture" and by extension, a characteristic mosaic or mottled pattern on the leaf. The symptoms were described as a mixture of dark green and light green areas, reflecting typical symptoms of a Tobacco mosaic virus (TMV) infection on tobacco (Fig. 1A). This new disease caused the plants to take on "a leaden-gray" color and the tobacco "was extremely bitter to the taste" (10).
| |
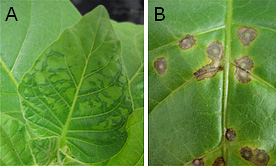
Fig. 1. Tobacco mosaic virus (TMV). (A) Systemic infections of Nicotiana tabacum cv. Turk plants showing TMV-associated mosaic. (B) Necrotic local lesions on N. tabacum Glurk leaf, demonstrating Holmes’ N-gene resistance following inoculation with TMV. Photo: K.-B. G. Scholthof. |
|
Colombian tobacco leaf was exported to Germany for cigars and may have been the source of TMV outbreaks in Europe. F. O. Holmes suggested that the center of origin of TMV was likely in South America, where N. glutinosa plants are resistant to TMV. This is a commonly accepted premise in plant pathology — resistance to a disease is found in the area native for the particular plant species (1). If so, it makes it more likely that TMV would have infected the cultivated tobacco crop in Colombia and subsequently transported to Europe. TMV is extremely stable in leaf material and touching a healthy tobacco plant can be sufficient to establish an infection (26,27).
Isolating the agent: TMV in the Netherlands. By 1857 tobacco growers in the Netherlands began to report that there was a new disease of tobacco. The new disease was not caused by fungi (molds) or bacteria. In 1879, Adolph Mayer, working in the Netherlands, investigated this disease and named it the mosaic disease of tobacco. He reported that the "the harm done by this disease is often very great ... and it has caused the cultivation of tobacco to be given up entirely" in certain places (17). Mayer reported that in the mid- to late-1800s the disease was known in the Netherlands and that he had observed it once in Germany, near Karlsruhe (17). In 1886, he described the development of the disease in infected plants, and that it could be transmitted to healthy plants by rubbing them with infected leaf sap (17). Mayer also showed that heating the sap at 80°C (175°F) for several hours killed the "infectious substance," but concluded it might be a type of bacterium. Several years later, Dmitrii Ivanowski reported the same disease in the Crimea. He determined that the agent was infectious after passage through a porcelain filter designed to retain bacteria. With these experiments he confirmed Mayer’s results on heat treatment and infectivity. From this Ivanowski concluded the infectious agent was a bacterium or perhaps a toxin (13).
In 1898, Martinus W. Beijerinck (Delft, the Netherlands) presented experimental evidence that the infection "is not caused by microbes, but by a contagium vivum fluidum" (2). The agent was infectious when diseased sap was passed through a Chamberland filter candle (Fig. 2) or by diffusion through agar (22,25,29). In contrast, bacteria were not filterable and were immobile in agar (contagium vivum fixum). Beijerinck showed the “virus” recapitulated the mosaic disease when inoculated to tobacco and was the first to use the term virus in a modern context. Thus, Beijerinck’s findings are considered the beginning of the study of the nature of the virus. More importantly, his research was predicated on a need: TMV was an economically important agricultural problem and practical solutions were required in order to control the disease to ensure continued production of tobacco. However, Beijerinck’s proof that TMV was a new infectious agent, clearly differing from bacterial or fungal infections, did not control the disease and commercial production of tobacco ended in the Netherlands.
 |
|
Fig. 2. Chamberland filter candles of the type used by Beijerinck. The unglazed porcelain filters are classified by type (L1, L3, L5) based on pore size. For example L1 lets all the bacteria filter through and is made for pre-filtration. The L3 type is very good for phage experiments. It retains bacteria but allows phage to pass through as filtrate. The L5 type has an even smaller pore size than L3.
These "Filtre Chamberland Systems Pasteur" filters were manufactured by Crane & Co. and purchased for 1.25 Dutch Guilders per piece. Photo and translation: K.-B. G. Scholthof. |
The Nature of the Virus
In the early 20th century, a primary question, following the discovery of bacteria and fungi, was to determine the nature of the virus — in particular, TMV. The study of the properties of viruses was of basic scientific interest and of practical value. The life-history of TMV from 1930-1960 has been elaborated by historians of science (4,5,14,15) and the plant virology community has provided accounts of TMV (9,22,23,25).
Koch’s postulates for a plant virus. Beijerinck had used two filtration techniques, a Chamberland filter (Fig. 2) and diffusion through agar, to show that TMV was infectious when rubbed onto healthy tobacco leaves. In his studies, a systemic infection was observed within two weeks (Fig. 1A). He had also shown that various bacteria were not the cause of the infection. Yet for the virus work to continue, it was necessary to develop tools to prepare pure cultures of the virus, to determine if there were strains or mutants, and to examine the host range of viruses. Although the working definition of a virus was mostly based on exclusion (not culturable, not bacteria, etc.) the early work followed the paradigms set by bacteriologists and mycologists under the loose umbrella of germ theory (16). This included being able to identify and characterize pure cultures of microbes in the laboratory and to reproduce the disease from pure culture, i.e., Koch’s postulates. In addition it was also necessary to determine if plants were infected since symptoms were not necessarily obvious on tobacco. This was a point of discussion in the early TMV papers — were the scientists observing the same disease caused by the same pathogen or a mixture of diseases or the effects of different environmental conditions (2,13,17,28)? Differing observations, or opinions, could be due to the virus not infecting the plant, a symptomless response of the plant to a virus infection, or the isolation of a new variant or a mixture of viruses that might cause much more severe symptoms. In particular, as many plant viruses cause similar symptoms, such as a dark green mosaic, and infect more than one host, it was critical to have confidence that a single virus was being studied. In parallel, a question developed as to the possibility that strains of an individual virus might exist or that a virus might mutate resulting in different symptoms or host range.
TMV Research in the United States
TMV, as I have previously argued, became a model system because tobacco was of economic importance (22). In the early 1900s, losses of 20-80% were common on plants infected with TMV, and for fresh market crops such as tomato and pepper, the fruits were often distorted or blemished which further reduced the wholesale and retail value. Developing new strategies to control virus diseases was a priority for American agriculture (3).
Beijerinck’s experiments recapitulated. In 1902 Albert F. Woods, a plant pathologist and physiologist, published the first report of TMV in the United States (28). Woods, a scientist with the Department of Agriculture, Bureau of Plant Industry (hereafter USDA) was charged with working on control and management of the disease. In his "Observations on the mosaic disease of tobacco," he reported on work performed from 1898-1900. He wrote that "the disease occurs more or less throughout the tobacco areas of this country and is widespread in Europe wherever tobacco is grown" (28). Yet Woods made little progress on understanding the nature of the disease and his opinion tended towards it being a physiological problem.
The early period: Key scientists. The scientists H. H. McKinney, Helen Purdy Beale, and Francis O. Holmes, each published a critical study in 1929 (12,18,20,21,22,23,25). In 1929, McKinney, a career employee of the USDA (19), was the first to report identifiable (phenotypic) TMV mutants on tobacco host plants (18). His practical application of this observation resulted in the development of cross protection, a method that uses a mild strain of virus to protect plants from a subsequent infection by a more severe (economically important) strain of the same virus (22,24). McKinney’s insight continues to be deployed today for protection of greenhouse crops (tomato and pepper) and field crops (squash and citrus). His finding formed the basis many decades later for the first demonstration of the use of transgenic plants to protect from virus infections (7). McKinney also developed the use of centrifugation for purification of plant viruses and published the results of using a prototype in July 1927 that was capable of spinning at 50,000 rpm (20).
Serology and Helen Purdy Beale. Helen Purdy Beale (1893-1976) used TMV to establish tools for plant virus serology that are now standard practice in research and diagnosis. Her professional career began when L. O. Kunkel hired her at the Boyce Thompson Research Institute in 1924, as she worked towards her Ph.D. at Columbia University. In 1929, Beale reported that serum collected from rabbits after serial injections of TMV-infected sap had antigenic properties not associated with healthy tobacco sap. This was important because it showed that TMV was a discrete pathogen and that TMV-specific antiserum could be used as a reagent to discriminate between TMV and other viruses. Beale developed the quantitative techniques of immunology and serology for the plant virus community in the early 20th century, yet it was not until the 1960s that her tools came into general use for diagnostics and experimentation. In a recent review, I have elaborated on her serological techniques and insight into using TMV serum to identify mutants and strains of TMV (23). Beale’s research was important both in determining that TMV was a substance that was not found in healthy plants, to unequivocally demonstrate a virus infection in plants (diagnostics), as well as ensuring that a single type of TMV was being used by Wendell M. Stanley during his purification and characterization of TMV. Beale remained at Boyce Thompson until she retired in 1960.
From local lesion assay to the N-gene: Francis O. Holmes. Francis O. Holmes (1897-1990) also shared McKinney’s interest in virus mutants, and was involved, along with Helen Purdy Beale, in characterizing the biological properties of TMV variants. Holmes showed that he had strains (mutants) of TMV, a spectrum of host plant species were available that were either susceptible or resistant to TMV infection (Fig. 1B), and the phenotype of the TMV mutants and the genetic effects of a host gene could be determined by observing the symptoms on plants. He also determined that the resistance responses could be temperature sensitive. By the mid-1930s, both in terms of inheritance and mechanistic processes, Holmes had shown that TMV was an entity that could be studied, manipulated, and understood within the context of the host-virus interaction.
As told by Creager (4) the major push towards solving the physicochemical properties of TMV occurred after L. O. Kunkel formed a plant virology group at Rockefeller Institute for Medical Research in Princeton, New Jersey (RIMR-Princeton). Holmes and Stanley were in the same laboratory at RIMR-Princeton in 1935 when Stanley’s physicochemical isolation of TMV crystals was hailed as a transformative event for biology – with results that were greeted with great enthusiasm by the public and scientists (4). In addition to this being a marker for the new biology – biochemistry and molecular biology, it also is a temporal marker of the division between basic and applied virology. Yet Holmes managed to straddle both worlds. In fact, his research resonates today: each semester his eponymous local lesion assay is used for undergraduate teaching demonstrations (Fig. 1B) and his manuscripts are regularly cited as tools and background for current research on molecular plant-virus interactions, virus movement, and breeding for improved resistance to agriculturally important crop plants.
Relevance of the TMV: N-gene interaction. In his 1938 Phytopathology paper, Holmes summarized the N-gene work in three points: "The protecting gene is inherited as a Mendelian dominant. Its identification by inoculation methods is readily accomplished. It is hoped, therefore, that further work on the incorporation of the gene into locally acceptable strains of tobacco and subsequent trials under field conditions may be left largely to those who are especially interested in the maintenance and improvement of varieties of tobacco" (11). Holmes’ research contributed to two important events in the late 1930s: Stanley’s findings on the nature of the virus and the development of TMV-resistance in crop plants. The development and significance of Holmes’ findings, within the context of agriculture and plant pathology in the United States in the early 20th century, is a key area of interest in revealing the history of TMV and plant pathology in the United States.
Conclusion, As Prelude
The research on TMV during the first decades of the 20th century was paradigm shifting, providing key elements to study and define the nature of a virus. Many of the techniques developed by Holmes, McKinney, and Beale have become commonplace for plant pathology and plant virology. Yet Tobacco mosaic virus has been an extraordinary tool in the past century, for research ranging from structural biology to host-plant resistance. During this period, TMV made the progression from a mysterious agent-of-disease to a reagent that is used to dissect the molecular mysteries of plant-pathogen interactions. In the coming years, TMV is sure to teach us a few more lessons about how viruses cause disease and, by extension, how to deploy new strategies for host-plant resistance or immunity to pathogens.
Additional Resources
Scholthof, K.-B. G. 2000 (updated in 2005). Plant disease lessons: Tobacco mosaic virus. The Plant Health Instructor. doi:10.1094/PHI-I-2000-1010-01.
Archives of the Delft School of Microbiology, The Beijerinck Museum, Delft University of Technology, the Netherlands.
Descriptions of Plant Viruses (DPVweb). From scientists at Rothamsted Research. Sponsored by the Assoc. of Applied Biologists and the Zhejiang Acad. of Agric. Sci., Hangzhou, China.
Literature Cited
1. Agrios, G. N. 2005. Plant Pathology, 5th Edn. Academic Press, New York, NY.
2. Beijerinck, M. W. 1898 [1968]. Concerning a contagium vivum fluidum as cause of the spot disease of tobacco leaves. Trans. J. Johnson. Pages 33-52 in: Phytopathological Classics, Number 7. J. Johnson, ed. American Phytopathological Society, St. Paul, MN.
3. Campbell, C. L., Peterson, P. D., and Griffith, C. S. 1999. The Formative Years of Plant Pathology in the United States. American Phytopathological Society, St. Paul, MN.
4. Creager, A. N. H. 2002. The Life of a Virus: Tobacco Mosaic Virus as an Experimental Model, 1930-1965. Univ. of Chicago, Chicago, IL.
5. Creager, A. N. H., Scholthof, K.-B. G., Citovsky, V., and Scholthof, H. B. 1999. Tobacco mosaic virus: Pioneering research for a century. Plant Cell 11:301-308.
6. Elferink, J. G. R. 1983. The narcotic and hallucinogenic use of tobacco in pre-Columbian Central America. J. Ethnopharmacol. 7:111-122.
7. Fulton, R. W. 1999. H. H. McKinney and cross protection. Pages 28-29 in: Tobacco Mosaic Virus: One Hundred Years of Contributions to Virology. K.-B. G. Scholthof, J. G. Shaw, and M. Zaitlin, eds. American Phytopathological Society, St. Paul, MN.
8. Goodspeed, T. H. 1954. The Genus Nicotiana: Origins, Relationships and Evolution of its Species in the Light of Their Distribution, Morphology and Cytogenetics. Chronica Botanica Co., Waltham, MA.
9. Harrison, B. D., and Wilson, T. M. A. 1999. Milestones in the research on tobacco mosaic virus. Phil. Trans. R. Soc. Lond. B 354:521-529.
10. Harrison, J. P. 1952. The evolution of the Colombian tobacco trade, to 1875. Hispanic Am. Hist. Rev. 32:163-174.
11. Holmes, F. O. 1938. Inheritance of resistance to tobacco-mosaic disease in tobacco. Phytopathology 28:553-561.
12. Holmes, F. O. 1929. Local lesions in tobacco mosaic. Bot. Gaz. 87:39-70.
13. Ivanowski, D. 1892 [1968]. Concerning the mosaic disease of the tobacco plant. Trans. J. Johnson. Pages 27-30 in: Phytopathological Classics Number 7. American Phytopathological Society, St. Paul, MN.
14. Kay, L. E. 1993. The Molecular Vision of Life: Caltech, The Rockefeller Foundation, and the Rise of the New Biology. Oxford Univ. Press, New York, NY.
15. Kay, L. E. 1986. W. M. Stanley's crystallization of the Tobacco mosaic virus, 1930-1940. Isis 77:450-472.
16. Kelman, A., and Peterson, P. D. 2002. Contributions of plant scientists to the development of the germ theory of disease. Microbes Infect. 4:257-260.
17. Mayer, A. 1886 [1968]. Concerning the mosaic disease of tobacco. Trans. J. Johnson. Pages 11-24 in: Phytopathological Classics Number 7. J. Johnson, ed. American Phytopathological Society, St. Paul, MN.
18. McKinney, H. H. 1929. Mosaic diseases in the Canary Islands, west Africa, and Gibraltar. J. Agric. Res. 39:557-578.
19. McKinney, H. H. 1972. Over fifty two years as a government servant in plant disease and virus research. Personal memoir. Author's files.
20. McKinney, H. H. 1927. Quantitative and purification methods in virus studies. J. Agric. Res. 35:13-38.
21. Purdy, H. A. 1929. Immunologic reactions with tobacco mosaic virus. J. Exper. Med. 49:919-935.
22. Scholthof, K.-B. G. 2004. Tobacco mosaic virus: A model system for plant biology. Annu. Rev. Phytopath. 42:13-34.
23. Scholthof, K.-B. G., and Peterson, P. D. 2006. The role of Helen Purdy Beale in the early development of plant serology and virology. Adv. Appl. Microbiol. 59:221-241.
24. Scholthof, K.-B. G., Scholthof, H. B., and Jackson, A. O. 1993. Control of plant virus diseases by pathogen-derived resistance in transgenic plants. Plant Physiol. 102:7-12.
25. Scholthof, K.-B. G., Shaw, J. G., and Zaitlin, M., eds. 1999. Tobacco Mosaic Virus: One Hundred Years of Contributions to Virology. American Phytopathological Society, St. Paul, MN
26. Valleau, W. D. 1942. Control of the common mosaic disease of tobacco by breeding. Phytopathology 32:1022-1025.
27. Valleau, W. D., and Johnson, E. M. 1927. The effect of a strain of tobacco mosaic on the yield and quality of Burley tobacco. Phytopathology 17:523-527.
28. Woods, A. F. 1902. Observations on the Mosaic Disease of Tobacco. Bull. No. 18. Bureau of Plant Industry, USDA, Washington, DC.
29. Zaitlin, M. 1998. The discovery of the causal agent of the tobacco mosaic disease. Pages 105-110 in: Discoveries in Plant Biology S.-D. Kung, and S. F. Yang, eds. World Scientific Publishing Co. Ltd., Hong Kong.
