Introduction
Increased use of foliar fungicides in corn has prompted members of the Corn Disease Working Group (CDWG) to explore potential impacts of this change in production practices, and determine if foliar fungicide use can be justified for uses other than disease management. Members of the CDWG are corn pathologists within the United States and Canada who meet annually to discuss important issues related to corn diseases. While much of this article results from these discussions, views expressed in this article may not represent the opinions of all members of the CDWG.
Hybrid corn (Zea mays) is an important commodity in the United States, with over 79.3 million acres in production per year since 2006 (47). Hybrid corn is susceptible to many foliar fungal diseases, including gray leaf spot (Cercospora zeae-maydis), northern leaf blight (Exserohilum turcicum), southern corn rust (Puccinia polysora), eyespot (Aureobasidium zeae), and others. Many foliar diseases can be managed through cultural practices such as planting hybrids with genetic resistance to disease and by reducing overwintering inoculum on residue through crop rotation and tillage. Foliar fungicides are also available to manage fungal diseases, and systemic fungicides have been labeled for use in corn since the 1990s. However, fungicides have traditionally been used only in seed or specialty corn production.
In 2007, fungicide use increased dramatically in hybrid corn, and now today’s corn crop is typically produced with higher fungicide inputs compared to five years ago. Increased fungicide use resulted from several coinciding factors, including an increase in the market price of corn, increased foliar diseases in parts of the United States, new fungicides becoming available for use on field crops, and fungicide manufacturers marketing of fungicides for use in hybrid corn.
From 1972 through 2005, the average corn price was around $2.00 per bushel (48). Over the past four years, corn prices have now averaged about $5.00 per bushel (Fig 1). Several factors have influenced these record high prices over the last few years, including expanding global markets, particularly in Asia, and also in the United States where legislation encourages use of alternative fuels, including ethanol made from corn. These factors have increased demand for corn and the increased value of corn has made this a profitable time for corn production. The increased value of corn also provides the economic justification for increased input costs, including increased fungicide use.
|
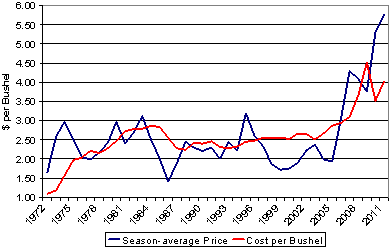
Fig. 1. Average price distribution (see 48) and production cost (see 15) per bushel of corn in the United States.
|
|
The need for disease management in corn has also increased in recent years. One reason is because crop production practices such as reduced tillage or no-till are increasing across the Corn Belt. Another reason is because the amount of Midwest acreage in continuous corn has also increased as producers capitalize on the aforementioned increasing demands for corn. Both trends have contributed to an increase in the amount of corn residue in many areas. This residue can serve as a source of primary inoculum for several important foliar diseases such as gray leaf spot, northern leaf blight, and eyespot. Traditionally, corn producers relied upon low-input cultural methods to manage these diseases, such as selecting hybrids with some level of genetic resistance, crop rotation, and tillage. Today, less emphasis is placed on selecting hybrids with strong disease resistance packages, and more emphasis is placed on selecting hybrids with high-yield potential. The net result is an increased reliance on fungicides for protecting the corn crop.
Changing weather patterns have also impacted corn growth and development, as well as altered disease and pest pressure (21). In recent years, there have been concerning outbreaks of southern rust, eyespot, northern leaf blight, and gray leaf spot in different corn production areas in the United States (2,41). It is predicted that leaf and root pathogens will be more problematic because of an overall increase in humidity and frequency of heavy rainfall events projected for many parts of the United States (10).
Individually, each of the various production factors can influence disease development in a given year. However, many corn producers throughout the Corn Belt are often dealing with several of these factors in a single field or area. Producers are in need of recommendations on how to effectively manage diseases in an era where cultural practices commonly used for disease management, such as crop rotation and tillage, may not be practical or economically feasible.
In the mid-2000s, quinone-outside inhibitor fungicides (QoI) fungicides were first labeled for use on corn, giving producers a newer, more effective fungicide class for disease management. These fungicides, commonly referred to as strobilurin fungicides, are now widely marketed in corn production for management of both biotic and abiotic stresses. Some fungicide manufacturers suggest these fungicides can increase yield even in the absence of disease. For example, benefits such as improved stalk strength and harvestability of corn are promoted to result from QoI fungicide use, and these claims are appealing to producers looking to retain standability of corn at harvest. As a result, many fungicide applications across the U.S. Corn Belt are now being made in anticipation of these perceived benefits, rather than being made in response to disease or a disease threat.
The willingness to spray one pesticide across many acres to control a wide range of pests was popularized in the 1990s with herbicides. Glyphosate-tolerant (Roundup Ready) soybeans were introduced in 1996, making broad-spectrum weed control easier and less expensive. Ten years later, more than 90% of U.S. soybean acres were treated with glyphosate for weed control (46). In 1998, Roundup Ready corn became available and now it is estimated that 50% of U.S. corn is treated with glyphosate (46). This widespread use has come at the expense of other traditional weed control tactics, such as the use of products with other modes of action, cultivation, and altered planting timings to suppress early season weed establishment. The repeated widespread use of glyphosate has inadvertently selected for tolerant weed species, and some formerly susceptible weed species have now developed glyphosate-resistant biotypes (35,55). Perhaps more importantly, glyphosate-tolerant crops have contributed towards the attitude of increased reliance on pesticides, with less emphasis being placed on scouting and more traditional integrated pest management (IPM-) based applications (35,55).
This relatively new attitude of eschewing IPM practices may also be contributing to the widespread use of foliar fungicides in corn production. Foliar fungicides have seen the most dramatic increase in use over the past 10 years compared to other pesticides in corn. For example, a 2002 CropLife Foundation summary of the National Pesticide Use Database did not even list field corn among crops that had significant acreage treated with a foliar fungicide (18). Furthermore, the need for and use of foliar fungicides in corn production was scarcely addressed in the 2002 United States Department of Agriculture (USDA) Field Corn Pest Management Strategic Plan (40). Foliar fungicides are now applied to more than 10 million acres of corn in the U.S. (CDWG members, personal communication), and often this is in the absence of a justifiable disease threat.
Oddly enough, a soybean disease also played a role that increased corn fungicide use. In 2005, chemical companies scrambled to stock foliar fungicides in the soybean- (and corn-) producing regions of the United States, in anticipation of the need to manage the recently-introduced disease soybean rust caused by the fungus Phakopsora pachyrhizi. Soybean rust was causing significant yield losses in Africa and South America (32,53) before it was first identified in the United States in November of 2004 (43). Because United States soybean varieties were not resistant to the new rust, there was intense concern that widespread fungicide applications would be needed to manage the disease in the soybean crop. Over the next several years, it became apparent that soybean rust would not be an annual threat to the major soybean producing regions of the United States, including those within the Corn Belt. However, this left pesticide manufacturers looking for a market for the fungicide stockpile. Shortly thereafter, fungicides were being promoted for use in field corn, for disease management and yield enhancement.
The combination of these factors contributed to growers routinely applying fungicides to corn in order to improve corn yields. Applications of fungicides, regardless of disease pressure, are often termed "insurance applications" and the practice ignores the principles of IPM.
Prior to 2008, limited replicated research data were available to aid developing recommendations for fungicide use in hybrid corn. Field research experiments demonstrated the efficacy of demethylation inhibitor (DMI) (e.g., triazole) fungicides against foliar diseases, such as gray leaf spot, but published research did not include results from QoI (strobilurin) fungicides, or quantify potential yield benefits in the absence of disease (33,50). Research on fungicide efficacy in hybrid corn is commonly reported in Plant Disease Management Reports (PDMR, formerly Fungicide & Nematicide [F&N]Tests), published by the American Phytopathological Society. A collection of these reports published between 2000 and 2010 (Table 1) show that prior to 2008, a total of 6 trials was published that examined the efficacy of fungicides on corn. Between 2008 and 2010, a total of 33 trials was published, which indicated the increased interest for research data on corn fungicides.
Table 1. Trials examining fungicide efficacy on hybrid dent corn published in Fungicide & Nematicide (F&N) Tests and Plant Disease Management Reports (PDMR) between 2000 and 2010. Only trials that included foliar disease ratings were included in the analysis.
| Authors |
State |
Year |
Publication |
| E. Stromberg and L. E. Flinchum |
Virginia |
2000 |
F&N 55:366 |
| E. Stromberg and L. E. Flinchum |
Virginia |
2001 |
F&N 56:FC6 |
| E. Stromberg and L. E. Flinchum |
Virginia |
2002 |
F&N57:FC91 |
| E. Stromberg and L. E. Flinchum |
Virginia |
2003 |
F&N 58:FC002 |
| E. L. Stromberg and C. C. Kenley |
Virginia |
2005 |
F&N 60:FC074 |
| T. Jackson |
Nebraska |
2006 |
F&N 61:FC041 |
| K. A. Ames and C. A. Bradley |
Illinois |
2008 |
PDMR 2:FC054 |
| A. K. Hagan |
Alabama |
2008 |
PDMR 2: FC092 |
T. A. Jackson, J. L. Behn, and
D. W. Miller |
Nebraska |
2008 |
PDMR 2: FC058 |
| C. Lee, P. Vincelli, and E. Dixon |
Kentucky |
2008 |
PDMR 2: FC096 |
P. A. Paul. A. L. Johnston, and
D. R. Mills |
Ohio |
2008 |
PDMR 2: FC018 |
| P. A. Paul and D. R. Mills |
Ohio |
2008 |
PDMR 2: FC038 |
P.A. Paul. A. L. Johnston, and
D. R. Mills |
Ohio |
2008 |
PDMR 2: FC054 |
| C. Rodriguez-Brljevich, J. M. Shriver, and A. Robertson |
Iowa |
2008 |
PDMR 2: FC097 |
| C. A. Bradley and K. A. Ames |
Illinois |
2009 |
PDMR 3: FC001 |
| C. A. Bradley and K. A. Ames |
Illinois |
2009 |
PDMR 3: FC002 |
| C. A. Bradley and K. A. Ames |
Illinois |
2009 |
PDMR 3: FC003 |
| J. A. Coulter |
Minnesota |
2009 |
PDMR 3: FC027 |
| A. P. Grybauskas and E. Reed |
Maryland |
2009 |
PDMR 3: FC013 |
| A. K. Hagan |
Alabama |
2009 |
PDMR 3: FC020 |
P. A. Paul, M. W. Wallhead, and
D. R. Mills |
Ohio |
2009 |
PDMR 3: FC092 |
P. A. Paul, M. W. Wallhead, and
D. R. Mills |
Ohio |
2009 |
PDMR 3: FC093 |
P. A. Paul, M. W. Wallhead, and
D. R. Mills |
Ohio |
2009 |
PDMR 3: FC094 |
P. A. Paul, M. W. Wallhead, and
D. R. Mills |
Ohio |
2009 |
PDMR 3: FC095 |
| J. M. Shriver, and A. E. Robertson |
Iowa |
2009 |
PDMR 3: FC014 |
| J. M. Shriver, and A. E. Robertson |
Iowa |
2009 |
PDMR 3: FC015 |
| E. L. Stromberg and C. C. Kenley |
Virginia |
2009 |
PDMR 3: FC017 |
| K. Wise, and G. Buechley |
Indiana |
2009 |
PDMR 3: FC097 |
| J. A. Coulter |
MN |
2010 |
PDMR 4: FC012 |
| T. A. Jackson, and J. L. Behn |
Nebraska |
2010 |
PDMR 4: FC084 |
| T. A. Jackson, C. M. Schleicher, and J. L. Behn |
Nebraska |
2010 |
PDMR 4: FC089 |
| T. A. Jackson, C. M. Schleicher, and J. L. Behn |
Nebraska |
2010 |
PDMR 4: FC090 |
| C. Lee, and P. Vincelli |
Kentucky |
2010 |
PDMR 4: FC019 |
| C. Lee, and P. Vincelli |
Kentucky |
2010 |
PDMR 4: FC020 |
| P.M. Phipps |
Virginia |
2010 |
PDMR 4: FC050 |
| A. E. Robertson, K. Pecinovsky, and L. Liu |
Iowa |
2010 |
PDMR 4: FC087 |
| K. Wise and G. Buechley |
Indiana |
2010 |
PDMR 4: FC091 |
| K. Wise and G. Buechley |
Indiana |
2010 |
PDMR 4: FC092 |
| K. Wise and G. Buechley |
Indiana |
2010 |
PDMR 4: FC093 |
Data from the F&N and PDMR reports were compiled from the 10-year span between 2000 and 2010 to analyze the effect of QoI fungicides on yield. In each trial, yield data were analyzed to determine the effect of now commercially available fungicides on yield. QoI fungicide application had a significant effect on yield in 18 of 39 trials, or 46% of trials, meaning over half of the time a fungicide did not significantly impact yield. However, statistical significance is often misunderstood by people outside of the scientific community, especially when yield increases are observed that are not deemed "significant." To determine the corn yield responses from a QoI fungicide application reported in F&N and PDMR trials, we subtracted the yield of the untreated control in a trial from the yield of each fungicide treatment in that same trial. Then, these yield responses from a total of 472 treatment comparisons were pooled into a common dataset. This dataset included only QoI fungicide treatments that were applied between growth stages V14 (14th leaf) and R5 (dent), with the majority of treatments applied between VT (tassel) and R2 (blister). Only trials that included foliar disease assessments were included in the data analyses.
Approximately 80% of the treatment comparisons had a positive yield response from a fungicide application, while 20% of treatments showed no or a negative yield response from a fungicide application (Fig. 2). Specific fungicide treatments increased yields in the majority of the trials. However, a positive yield response from a fungicide application does not necessarily result in a positive economic benefit. Foliar fungicides are additional corn production inputs, and for applications to be profitable, corn producers must see a yield increase substantial enough to at least pay for the application. This is economic "break-even" value depends on costs associated with the fungicide application method (air or ground), product cost, as well as the market price of corn (Table 2).
|
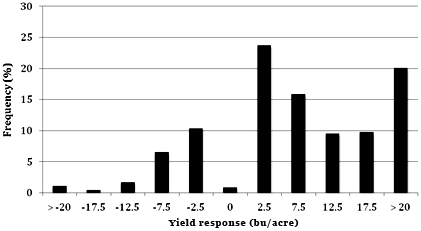
Fig. 2. Frequency distribution of corn yield response to fungicide. Data represent 472 treatment comparisons from trials published from 2000 to 2010 in Fungicide & Nematicide Tests or PlantDisease Management Reports. Yield response is calculated as the yield from the untreated control in a trial, subtracted from the yield of each fungicide treatment in that trial. Values on the X-axis represent the midpoint of each category.
|
|
Table 2. Examples of break-even corn yield increases (bu/acre) needed to pay for the cost of a fungicide application, based on market price and application cost. "Application cost" includes cost for fungicide product and application method.
| Corn price ($/bu) |
Application cost ($/acre) |
| $20 |
$24 |
$28 |
$32 |
| $2.00 |
10.0x |
12.0 |
14.0 |
16.0 |
| $3.00 |
6.7 |
8.0 |
9.3 |
10.7 |
| $4.00 |
5.0 |
6.0 |
7.0 |
8.0 |
| $5.00 |
4.0 |
4.8 |
5.6 |
6.4 |
| $6.00 |
3.3 |
4.0 |
4.7 |
5.3 |
| $7.00 |
2.9 |
3.4 |
4.0 |
4.6 |
| $8.00 |
2.5 |
3.0 |
3.5 |
4.0 |
x Bushels per acre required to "break even" at the given corn price and total fungicide cost.
The yield increase per acre needed to break-even from a fungicide application averaged approximately 6 bushels per acre (bu/acre) over much of the United States in 2009 and 2010. The F&N and PDMR data indicate that only 48% of 472 treatments resulted in a yield response that met or exceeded the economic break-even value of 6 bu/acre, even though 80% of treatments resulted in a positive yield response.
Compiling and analyzing published data allows researchers to examine the effect of a fungicide on yield over many locations and comparisons, but is limited by the amount of published data, and does not take into account data from trials that may not have been published in these or other journals. Therefore, regional corn fungicide yield responses were pooled without regard to publication status from research trials conducted by corn pathologists in the CDWG (Table 3) from 2008 to 2010 (613 treatment comparisons) in order to observe the effects of fungicide on yield. A portion of this data set was analyzed in the PDMR and F&N dataset of 472 treatment comparisons; however, previously unpublished data was also included in the CDWG dataset. Results from the CDWG data indicated that a yield response that met or exceeded 6 bu/acre occurred 45% of the time when a single fungicide application was made between the V15 and R2 growth stages of corn. This response rate is similar to the 48% yield response observed from F&N and PDMR data.
Table 3. Contributors of data submitted to regional corn fungicide summary of the Corn Disease Working Group. Data are included only for trials in which disease severity ratings were submitted.
| Contributor |
Affiliation |
Year trials submitted |
| T. Allen |
Mississippi State University |
2008 |
| G. Bergstrom |
Cornell University |
2010 |
| C. Bradley |
University of Illinois |
2008, 2009, 2010 |
| P. Esker |
University of Wisconsin |
2008, 2009, 2010 |
| A. Grybauskas |
University of Maryland |
2008, 2009, 2010 |
| T. Jackson |
University of Nebraska |
2009, 2010 |
| D. Jardine |
Kansas State University |
2008, 2010 |
| D. Malvick |
University of Minnesota |
2008, 2010 |
M. McMullen, G. Endres,
J. Ransom |
North Dakota State University |
2009, 2010 |
| L. Osborne |
South Dakota State University |
2008, 2009, 2010 |
| P. Paul |
Ohio State University |
2008, 2009, 2010 |
| A. Robertson |
Iowa State University |
2008, 2009, 2010 |
| E. Stromberg |
Virginia Polytechnic Institute and State University |
2008 |
| L. Sweets |
University of Missouri |
2008 |
| A. Tenuta |
Ontario Ministry of Agriculture, Food & Rural Affairs |
2009, 2010 |
| P. Vincelli |
University of Kentucky |
2008 |
| K. Wise |
Purdue University |
2008, 2009, 2010 |
Foliar disease can influence the yield response obtained from a fungicide application. This often increases the odds of meeting or exceeding the economic break-even value for a fungicide application. For example, in Nebraska where disease pressure is typically high, yield responses from trials between 2005 and 2009 revealed that approximately 90% of fungicide treatments resulted in a positive yield increase when applied between the VT and R4 (dough) growth stages (Fig. 3).
|

Fig. 3. Frequency distribution of corn yield response to fungicide in Nebraska, in the presence of relatively high disease. Data represent 53 treatment comparisons from trials published from 2006 to 2010 in Plant Disease Management Reports. Yield response is calculated as the yield from each untreated control in a trial, subtracted from the yield of the fungicide treatment. Values on the X-axis represent the midpoint of each category.
|
|
Conversely, low disease pressure environments usually resulted in less consistent positive yield responses from a fungicide application. For example, yield response data from Minnesota during 2009 and 2010 revealed that only one of 35 treatments (3%) resulted in a yield response greater than 6 bu/acre when the fungicide application occurred between the V14 and R2 growth stages (11,12). In these trials, final disease severity of northern leaf blight averaged less than 1%; and no other noticeable disease was observed. Yield response data submitted by CDWG members reinforce this concept. In the CDWG dataset for 2008, 2009 and 2010, the average yield response from a fungicide application was 9.6 bu/acre when final foliar disease severity was greater than 5% at R5 (Table 4). However, in trials conducted under low disease pressure (less than 5% foliar disease severity), the average yield response from a fungicide application was 1.5 bu/acre (Table 4).
Table 4. Corn yield response to QoI fungicides from 2008 to 2010, as determined by data submitted to the regional corn fungicide summary of the Corn Disease Working Group. Data are pooled across all treatments that included a QoI fungicide applied between V15 and R3 growth stages. Data include only trials where disease severity ratings were submitted.
| Disease severity of untreated (%) |
Mean yield response (bu/acre) |
Total treatments |
Percent of treatments with break-even yield response of 6 bu/acre |
| < 5 |
1.5 |
347 |
31.6 |
| > 5 |
9.6 |
266 |
59.0 |
| Total |
|
613 |
|
|
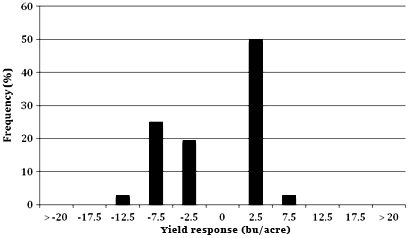
Fig. 4. Frequency distribution of corn yield response to fungicide in Minnesota, in the presence of relatively low disease. Data represent 36 treatment comparisons from trials published from 2009 to 2010 in Plant Disease Management Reports. Yield response is calculated as the yield from each untreated control in a trial, subtracted from the yield of the fungicide treatment. Values on the X-axis represent the midpoint of each category.
|
|
The majority of fungicides marketed for use on corn contain at least one active ingredient with the QoI mode of action, which are effective against a broad spectrum of fungal pathogens (3). This mode of action targets the cytochrome bc complex in the mitochondrial respiration pathway and blocks electron transport in fungi and plants. In fungi, this prevents spore germination and can reduce mycelial growth (3). In plants, QoI fungicides may act to increase water and nitrogen use efficiency, improve chlorophyll retention and delay senescence, and increase antioxidant activity (5,9,19,20,42,49,52,53). These physiological responses may occur in the absence of disease, and have been reported to increase yields in wheat and barley (9). Yield increases due to physiological plant effects have also been promoted in corn and soybean despite research in soybean and other crops that indicates physiological effects are inconsistent, or do not always result in a measurable yield increase (5,14,28,34,36,45). Analysis of wheat trials conducted between 1994 and 2010, in which no significant foliar disease was present, revealed an average yield response from a QoI fungicide application only resulted in profit 7% of the time. When wheat diseases were present, however, over 50% of the 100 comparisons met the break-even value for a yield increase (51). Although research in field crops indicates that yield benefits from strobilurin fungicides are inconsistent in the absence of disease, in many cases, low-disease environments are a focus for marketing efforts promoting fungicide application.
Another promoted advantage for foliar fungicide application is improving harvestability of corn. In other words, foliar fungicide applications may improve the standability of corn, thus helping reduce harvest loss due to lodging. Standability is important in corn production, since lodged corn may not be harvestable, result in significant yield loss, and/or it may increase the amount of time needed to harvest a field. Therefore, the possibility that a fungicide may improve standability appeals to corn producers. Additionally, the delayed senescence, or "greening effect," observed after the application of some QoI fungicides can lengthen the harvest season, which becomes problematic for growers trying to harvest many acres, or when harvest is delayed by inclement weather.
The general response of standability to fungicides is inconsistent, similar to yield responses measured in the absence of foliar disease. Lodging from stalk rot is one factor that can contribute to standability. However, research results are inconsistent for the effects of fungicide applications on stalk rot severity in corn. Trials conducted in Illinois from 2008 to 2010 demonstrated that a foliar fungicide application reduced incidence of stalk rot in trials where foliar disease pressure was high (16). Severe foliar disease during reproductive stages of corn development can reduce the photosynthetic area needed by the plant to drive grain fill. When plants are stressed and photosynthetic area is reduced, the corn plant will draw sugars from the stalk and direct them to the developing ear. This can compromise stalk integrity and lead to problems with stalk rots if foliar disease is severe (13). However, the presence of stalk rot does not necessarily mean lodging will occur, which can further complicate the justification behind fungicide applications to improve standability. Furthermore, in the many fields where foliar diseases are managed through cultural practices such as hybrid selection and crop rotation, it has not been established that standability is predictably improved through the application of fungicide. Finally, under some environmental conditions QoI fungicides may delay senescence, which can increase the time needed for corn to dry down to acceptable moisture for grain storage. This greening effect can result in delayed harvest or the harvest of high-moisture corn. This can ultimately reduce profitability when producers need to pay for dryer costs to prepare grain for storage.
Research indicates that even if fungicide applications have the potential to increase corn yield or improve standability in the absence of disease, they may not be profitable. In contrast, when fungicides are applied when disease is present or when the risk of disease is high, the likelihood for a positive economic response greatly increases. High disease risk practices include planting hybrids susceptible to foliar disease, planting continuous corn, minimum or no-till production systems, irrigated fields, and planting corn late. Weather conditions as corn enters reproductive stages of development will also influence disease risk. Humid and wet weather prior to and during early and mid-reproductive growth stages will increase the likelihood of foliar disease development. A combination of these factors increases the probability that disease will develop in a given year and consequently the likelihood for a benefit from a fungicide application.
"Insurance" applications of fungicides have potential consequences, not only from the economic perspective (use of additional inputs in corn production), but from the biological impact on fungal populations. The QoI fungicides are classified by the Fungicide Resistance Action Committee (FRAC) as high-risk for resistance development. Applications of these fungicides may increase selection pressure that could lead to shifts in fungal sensitivity to QoI fungicides. There are currently over 40 fungal pathogens with resistance to this fungicide class worldwide, and fungicide resistance has been detected within a pathogen as quickly as two years after widespread use of the QoI fungicides (17). Moreover, QoI resistance has recently been confirmed for the frogeye leaf spot pathogen, Cercospora sojina after just a few years of QoI fungicide use on soybean (6).
A proactive approach to identify shifts in sensitivity to QoI fungicides is to monitor fungal populations. For example, a sensitivity monitoring program has been established for the causal agent of gray leaf spot (7), which can help detect shifts in sensitivity and resistance development before widespread chemical control failures occur. However, should resistance develop, the loss of effective fungicides for disease management could result in situations where adequate disease control cannot be achieved. A better understanding of the impact of QoI fungicides in corn production is needed in order to maintain the long-term efficacy of these compounds in field crop production.
In addition to the development of fungicide resistance, there are additional side effects of widespread fungicide use in corn. Under certain conditions that are not well understood, the use of pesticides can exacerbate insect or mite pressure (31). Specifically, the use of fungicides in corn can reduce populations of entomopathogenic fungi, leaving the crop more at risk for insect or mite outbreaks (44). Recently, fungicide use has been tied to aphid flare-ups in several cropping systems (1,29,30,38). This consequence of fungicide use could impact crop production by eventually facilitating the need to manage insects that were not problematic prior to the widespread use of fungicides.
The dramatic increase in use of foliar fungicides also is raising environmental concerns in corn production areas. QoI fungicides, while defined as reduced-risk (3), are toxic to several aquatic species. For example, commonly used QoI fungicides have demonstrated acute toxicity to frog tadpoles (4,27), freshwater mussels (8), freshwater algae and water fleas (39) in laboratory studies. The potential side effects of increased fungicide applications on biological systems needs to be investigated further, and caution must be used to prevent disruption of natural systems due to misuse or overuse of fungicides.
The link between profitability of fungicides and the presence of significant disease pressure has been established. Therefore, scouting for presence and level of disease, correct disease identification, and optimum application timing for disease management should be emphasized when considering a fungicide application. In many cases, economic thresholds to justify fungicide application have not been developed for specific foliar diseases, and may be difficult to establish due to the impact of hybrid, cropping system, and environment on disease development. In these cases, it is difficult to use the traditional IPM approach of a threshold-based spray decision, and growers may be persuaded to apply fungicides as "insurance" for the crop. However, research-based results indicate that fungicide use in corn is most profitable when there is high risk for foliar disease development, and disease develops to levels that warrant management. For future longevity and profitability of fungicides in corn, the decision to apply a fungicide should be based on disease factors, and not based on presumed yield enhancements that might occur in the absence of disease.
The authors thank G. Shaner for help in compilation and analysis of 2008 to 2010 CDWG data, and D. Teska for aid in compilation of F&N and PDMR data. G. Shaner and P. Vincelli are also thanked for helpful comments and revisions to the manuscript.
1. Abney, M. R., Ruberson, J. R., Herzog, G. A., Kring, T. J., Steinkraus, D. C., and Roberts, P. M. 2008. Rise and fall of cotton aphid (Hemiptera: Aphididae) populations in southeastern cotton production systems. J. Econ. Entomol. 101:23-35.
2. Allen, T., and Monfort, S. 2010. Southern corn rust in fields. Online. Delta Farm Press, Clarksdale, MS.
3. Bartlett, D. W., Clough, J. M., Godwin, J. R., Hall, A. A., Hamer, M., and Parr-Dobrzanski, B. 2002. The strobilurin fungicides. Pest Manag. Sci. 58:649-662.
4. Belden, J., McMurry, S., Smith, L., and Reilley, P. 2010. Acute toxicity of fungicide formulations to amphibians at environmentally relevant concentrations. Environ. Toxicol. and Chem. 29:2477-2480.
5. Bertelsen, F. R., de Neergaard, E., and Smedegaard-Petersen, V. 2001. Fungicidal effects of azoxystrobin and epoxiconzole on phyllosphere fungi, senescence and yield of winter wheat. Plant Pathol. 50:190-205.
6. Bradley, C. A. 2010. Frogeye leaf spot pathogen with reduced sensitivity to fungicides found in Tennessee soybean field. University of Illinois Extension Bulletin. 24:172.
7. Bradley, C. A., and Pedersen, D. K. 2011. Baseline sensitivity of Cercospora zeae-maydis to quinone outside inhibitor fungicides. Plant Dis. 95:189-194.
8. Bringolf, R. B., Cope, W. G., Eads, C. B., Lazara, P. R., Barnhart, M. C., and Shea, D. 2007. Acute and chronic toxicity of technical-grade pesticides to glochidia and juveniles of freshwater mussels (Unionidae). Environ. Toxicol. Chem. 26:2086-2093.
9. Bryson, R. J., Leandro, L., and Jones, D. R. 2000. The physiological effects of kresoxim-methyl on wheat leaf greenness and the implications for crop yield. Pages 739-746 in: Proc. of the BCPC Conf. on Pests and Diseases, Brighton, UK, 13-16 November 2000. BCPC, Farnham, Surrey, UK..
10. Coakley, S. M., Scherm, H., and Chakraborty, S. 1999. Climate change and plant disease management. Ann. Rev. Phytopathol. 37: 399-426.
11. Coulter, J. A., 2009. Corn response to Headline fungicide application rate and timing in Minnesota, 2008. Plant Dis. Manag. Rep. 3:FC027.
12. Coulter, J. A., 2010. Response of corn hybrids with differing maturity to foliar fungicide in Minnesota, 2009. Plant Dis. Manag. Rep. 3:FC027.
13. Dodd, J. L. 1977. A photosynthetic stress translocation balance concept of corn stalk rot. Proc. Annu. Corn Sorghum Res. Conf. 32:122-130.
14. Dorrance, A. E., Cruz, C., Mills, D., Bender, R., Koenig, M., LaBarge, G., Leeds, R., Mangione, D., McCluer, G., Ruhl, S., Siegrist, H., Sundermeier, A., Sonnenberg, D., Yost, J., Watters, H., Wilson, G., and Hammond, R. B. 2010. Effect of foliar fungicide and insecticide applications on soybeans in Ohio. Online. Plant Health Progress doi:10.1094/PHP-2010-0122-01-RS.
15. Duffy, M. 2010. Ag decision maker: Historical Costs of Crop Production. Online. http://www.extension.iastate.edu/agdm/crops/pdf/a1-21.pdf
16. Ebelhar, S. A., Hart, C. D., and Bradley, C. A. 2010. Corn insecticide seed treatment and foliar fungicide effects on corn response to fertilizer nitrogen. Online. Proc. Illinois Fertilizer Conf., Dept. of Crop Sci., Univ. of Illinois Ext., Urbana-Champaign, IL.17. FRAC. 2011. Fungicide Resistance Action Committee (FRAC). Online. Croplife Intl., Brussels, Belgium.
18. Gianessi, L., and Reigner, N. 2006. Pesticide use in U.S. crop production. 2002. Online. Crop Protection Research Institute, CropLife Foundation, Washington, DC.
19. Glaab, J., and Kaiser, W. M. 1999. Increased nitrate reductase activity in leaf tissue after application of the fungicide kresoxim-methyl. Planta 207:442-448.
20. Grossman, K., and Retzlaff, G. 1997. Bioregulatory effects of the fungicide strobilurin kresoxim-methyl in wheat (Triticum aestivum). Pestic. Sci. 50:11-20.
21. Hatfield, J., Boote, K., Fay, P., Hahn, L., Izaurralde, C., Kimball, B. A., Mader, T., Morgan, J., Ort, D., Polley, W., Thomson, A., and Wolfe, D. 2008. The effects of climate change on agriculture, land resources, water resources, and biodiversity: Agriculture. U.S. Climate Change Science Program and the Subcommittee on Global Change Research, Washington, DC.
22. Jackson, T. A. 2006. Evaluation of foliar fungicides on gray leaf spot of corn in Nebraska, 2005. F&N Tests. 61:FC041.
23. Jackson, T. A., Behn, J. L., and Miller, D. W. 2008. Evaluation of foliar fungicides on leaf disease of corn in Nebraska, 2007. Plant Dis. Manag. Rep. 4:FC084.
24. Jackson, T. A., and Behn, J. L. 2010. Evaluation of foliar fungicides on gray leaf spot of corn in Nebraska, 2009. Plant Dis. Manag. Rep. 4:FC084.
25. Jackson, T. A., Schleicher, C. M., and Behn, J. L. 2010. Evaluation of foliar fungicides on gray leaf spot of field corn in Nebraska, 2009. Plant Dis. Manag. Rep. 4:FC090.
26. Jackson, T. A., Schleicher, C. M., and Behn, J. L. 2010. Foliar fungicides efficacy on gray leaf spot of field corn in Nebraska, 2009. Plant Dis. Manag. Rep. 4:FC090.
27. Johansson, M., Piha, H., Kylin, H., and Merila, J. 2006. Toxicity of six pesticides to common frog (Rana temporaria) tadpoles. Environ. Toxicol. Chem. 25:3164–3170.
28. Khan, M. F. R., and Carlson, A. L. 2009. Effect of fungicides on sugar beet yield, quality, and postharvest respiration rates in the absence of disease. Online. Plant Health Progress doi:10.1094/PHP-2009-1019-01-RS.
29. Koch, K. A., Potter, B. D. and Ragsdale, D. W. 2010. Non-target impacts of soybean rust fungicides on the fungal entomopathogens of soybean aphid. J. Invertebrate Pathology. 103:156-164.
30. Lagnaoui, A., and Radcliffe, E. B. 1998. Potato fungicides interfere with entomopathogenic fungi impacting population dynamics of green peach aphid. Amer. J. Potato Res. 75: 19-25.
31. Latteur, G., and Jansen, J. P. 2002. Effects of 20 fungicides on the infectivity of conidia of the aphid entomopathogenic fungus Erynia neoaphidis. BioControl 47: 435-444.
32. Levy, C. 2005. Epidemiology and Chemical Control of Soybean Rust in Southern Africa. Plant Dis. 89:669-674.
33. Munkvold, G. P., Martinson, C. A., Shriver, J. M., and Dixon, P. M. 2001. Probabilities for profitable fungicide use against gray leaf spot in hybrid maize. Phytopathology 91:477-484.
34. Nason, M. A., Farrar, J., and Bartlett, D. 2007. Strobilurin fungicides induce changes in photosynthetic gas exchange that do not improve water use efficiency of plants grown under conditions of water stress. Pest Manag. Sci. 63:1191-1200.
35. National Research Council. 2010. The Impact of Genetically Engineered Crops on Farm Sustainability in the United States. The National Academies Press, Washington, DC.
36. Nelson, K. A., Motavalli, P. P., Stevens, W. E., Dunn, D., and Meinhardt, C. G. 2010. Soybean response to preplant and foliar-applied potassium chloride with strobilurin fungicides. Agron. J. 102:1657-1663.
37. Newman, M. A., and Bradley, C. A. 2011. Soybean pathogen found to be resistant to fungicides. Proc. Annu.Southern Soybean Dis. Workshop, p 9.
38. Nielsen, C., and Hajek, A. E. 2005. Control of invasive soybean aphid, Aphis glycines (Hemitera: Aphidiidae), populations by existing natural enemies in New York State, with emphasis on entomopathogenic fungi. Environ. Entomol. 34:1036-1047.
39. Ochoa-Acuna, H. G., Bialkowski, W., Yale, G., and Hahn, L. 2009. Toxicity of soybean rust fungicides to freshwater algae and Daphnia magna. Ecotoxicology 18:440-446.
40. Pike, D. R. 2002. Field Corn Pest Management Strategic Plan North Central Region. Online. North Central Integrated Pest Management Center, CSREES, Washington, DC. 41. Robertson, A. 2009. Eyespot and gray leaf spot occurring in corn. Integrated Crop Management Newsletter. Online. Iowa State University, Ames, IA.
42. Ruske, R. E., Gooding, M. J., and Jones, S. A. 2003. The effects of triazole and strobilurin fungicide programmes on nitrogen uptake, partitioning, remobilization and grain N accumulation in winter wheat cultivars. J. Agric. Sci. 140:395-407.
43. Schneider, W., Hollier, C. A., Whitam, H. K., Palm, M. E., McKemy, J. M., Hernandez, J. R., Levy, L., and DeVries-Paterson, R. 2005. First report of soybean rust caused by Phakopsora pachyrhizi in the continental United States. Plant Dis. 89:774.
44. Smitley, D. R., Kennedy, G. G., and Brooks, W. M. 1986. Role of the entomopathogenic fungus, Neozygites floridana, in population declines of the two-spotted spider mite, Tetranychus urticae, on field corn. Entomologia Experimentalis et Applicata. 41:255-264.
45. Swoboda, C., and Pedersen, P. 2009. Effect of fungicide on soybean growth and yield. Agron. J. 101:352-356.
46. United States Department of Agriculture. 2007. National Agricultural Statistics Service. Agricultural Chemical Use Database. Online. USDA, Washington, DC.47. United State Department of Agriculture. 2010. National Agricultural Statistics Service. Online. USDA, Washington, DC.
48. United State Department of Agriculture. 2010. Economics, Statistics and Market Information System (ESMIS). Online. USDA, Washington, DC.
49. Venacio, W. S., Rodrigues, M. A. T., Begliomini, E., and de Souza, N. L. 2003. Physiological effects of strobilurin fungicides on plants. Ponta Grossa 9:59-68.
50. Ward, J. M. J., Laing, M. D., and Nowell, D. C. 1997. Chemical control of maize grey leaf spot. Crop Prot. 16:265-271.
51. Weisz, R., Cowger, C., Ambrose, G., and Gardner, A. 2011. Multiple mid-Atlantic field experiments show no economic benefit to fungicide application when fungal disease is absent in winter wheat. Phytopathology 101:323:333.
52. Wu, Y., and von Tiedemann, A. 2002. Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ. Pollut. 116:37-47.
53. Wu, Y., and von Tiedemann, A. 2001. Physiological effect of azoxystrobin and epoxiconazole on senescence and the oxidative status of wheat. Pest. Biochem. Physiol. 71:1-10.
54. Yorinori, J. T., Paiva, W. M., Frederick, R. D., Costamilan, L. M. and Bertagnolli, P. F. 2005. Epidemics of Soybean Rust (Phakopsora pachyrhizi) in Brazil and Paraguay from 2001 to 2003. Plant Dis. 89:675-677.
55. Young, B. 2006. Changes in herbicide use patterns and productions practices resulting from glyphosate-resistant crops. Weed Tech. 20:301-307.
