Pathogen Biology
The pathogen exists in all major sunflower-producing regions worldwide (Friskop and Markell 2016).
Puccinia helianthi can infect all 61 known
Helianthus species in North America; many of which can now be found in other areas of the world (Friskop et al. 2011, Gulya et al. 1997). This includes all cultivated sunflower (H. annuus), ornamental sunflower, and wild sunflower.
P. helianthi is an obligate biotroph, meaning that it can only survive on a living host (Friskop and Markell 2016). It is a macrocyclic rust fungus, meaning that it produces five spore stages: basidiospores, pycniospores (spermatia), aeciospores, urediniospores, and teliospores (D'Arcy et al. 2001, Friskop and Markell 2016). The pathogen is autoecious, meaning that it only needs a single host to complete its life cycle. Notably, many rust pathogens are heteroecious, meaning that two different and unrelated hosts are needed to complete the life cycle (such as
P. graminis f. sp. tritici, the causal agent of stem rust of wheat/barley and barberry) (Schumann and Leonard 2000). Consequently, the complete life cycle of P. helianthi may occur more frequently than that of many other rusts, as all stages occur on sunflower.
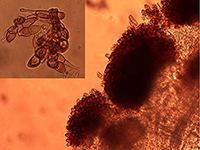
Figure 1. Telia and teliospores (inset) of
Puccinia helianthi (Bob Harveson, UNL).
| 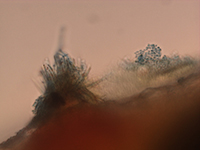
Figure 2. Pycniospores and receptive hyphae of
Puccinia helianthi
(Bob Harveson, UNL).
| 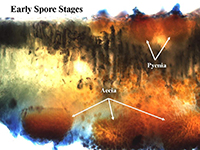
Figure 3. Cross-section of a sunflower leaf showing aecia and pycnidia of
Puccinia helianthi (Bob Harveson, UNL).
| 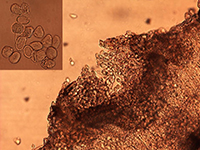
Figure 4. Urediniospores (inset) liberated from an aecia of Puccinia helianthi (Bob Harveson, UNL).
|
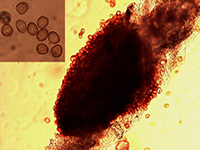
Figure 5. Urediniospores (inset) liberated from a uredinia of Puccinia helianthi (Bob Harveson, UNL).
| 
Figure 6. Pycnial lesions of
Puccinia helianthi on the cotyledon of a sunflower (Sam Markell, NDSU).
| 
Figure 7. Pycnial lesion of Puccinia helianthi on a sunflower leaf (Sam Markell, NDSU).
| 
Figure 8. Aecia of Puccinia helianthi on a sunflower leaf, directly opposite the pycnial lesion in Figure 7 (Sam Markell, NDSU).
|
The complete life cycle includes both a sexual and asexual phase, creating a pathogen that has a high level of genetic diversity and explosive epidemic potential (Harveson 2010b, Markell et al. 2016). The life cycle begins in the telia, which produces teliospores where karyogamy (fusion of haploid nuclei to generate diploid nuclei) takes place (Fig. 1) (D'Arcy et al. 2001, Friskop and Markell 2016). Meiosis then follows, producing four haploid basidospores of two different mating types, termed "+" and "-". Basidiospores infect sunflower and produce a pycnium with receptive hyphae and pycniospores on the top side of a leaf or cotyledon (Fig. 2). The cycle continues only when pycniospores from a different mating type contact and fertilize the pycnium. Pycniospore movement resulting in cross-fertilization may occur by several mechanisms, including when pycniospores are transferred via insects in the sticky honeydew the spores are produced in, by rain splash, or by physical contact. Once fertilized, dikaryotic mycelium is produced; a nuclear state that remains until the sexual cycle is repeated again. Mycelium grows vertically through the leaf tissue to produce an aecium opposite the pycnia (Fig. 3). Aeciospores liberated from the aecium then infect sunflowers to produce pustules containing the characteristic cinnamon-brown urediniospores (Figs. 4 and 5). Urediniospores that infect the sunflower give rise to more uredinia pustules containing uredinispores. This is commonly called the "repeating stage." This repeating stage can happen many times in the same growing season, rapidly increasing the amount of spores, which can lead to an explosive epidemic locally and spread of the pathogen great distances to other commercial fields or stands of wild sunflowers. It is notable that in production regions with mild climates (such as in Australia), urediniospores can overwinter and serve as a source of inoculum in the next growing season. Towards plant maturity and triggered by environmental conditions (primarily cooler temperatures), teliospores are produced in telia (Fig. 1). The importance of telia in this cycle is twofold: as a robust overwintering structure and as a method of sexual reproduction, the latter of which can result in great genetic diversity and a high likelihood of generating new "races" (Markell et al. 2016).
Races (or pathotypes) of
P. helianthi have been identified on all sunflower-producing continents (Friskop et al. 2015b, Jing et al. 2014, Markell et al. 2016, Meyer et al. 2020, Moreno et al. 2011, Sendall et al. 2006, Tan 2010). The nomenclatural term race is used in plant pathology to differentiate a species based on nonmorphological factors, such as virulence to specific resistance genes, host range, or symptom expression (D'Arcy et al. 2001). In the case of
P. helianthi, races are determined by the pathogen's ability to cause infection on a combination of known resistance genes or alleles (Friskop et al. 2015b, Markell et al. 2016, Meyer et al. 2020, Sendal et al. 2006). Succinctly, a pathogen's virulence is determined by evaluating its ability to cause infection on a host plant with known resistance gene(s) or allele(s) (commonly called a differential line). Races are named based on the combination of differential lines on which the pathogen confers virulence; races are essentially a shorthand nomenclature for virulence of the pathogen. Race names may change as differential lines vary over time as differentials are added or removed and should not be considered static subdivisions of any pathogen (Gilley et al. 2020). Further, race names do not always translate internationally, as various differential lines may not be relevant to all production regions.

Figure 9. Decaying sunflower cotyledon with numerous aecia of Puccinia helianthi (Sam Markell, NDSU).
| 
Figure 10. Aecial cups of Puccinia helianthi on sunflower cotyledon (Sam Markell, NDSU).
| 
Figure 11. Aecia of Puccinia helianthi on the vein of a sunflower leaf (Sam Markell, NDSU).
| 
Figure 12. Uredinia of Puccinia helianthi with chlorotic yellow halos and dusty cinnamon-brown urediniospores (Andrew Friskop, NDSU).
| 
Figure 13. Severely infected sunflower leaf with uredinia of Puccinia helianthi not surrounded by chlorotic halos (Sam Markell, NDSU).
|
Symptoms and Signs
Four of the five spore stages of the sunflower rust pathogen's life cycle are visible to the unaided eye (Friskop et al. 2011a, Harveson 2010a, Markell et al. 2018). The earliest visible sign is small (often 6 mm or less) yellow to orange pycnial lesions. Pycnial lesions are embedded on the upper side of lower sunflower leaves or cotyledons and may be surrounded by a chlorotic halo (Figs. 6 and 7). The second stage of the life cycle, aecia, are visible on the opposite side of the leaf to the pycnia on the underside of leaves or cotyledons (Figs. 8 and 9). Aecia are a cluster of orange cups filled with orange spores (Fig. 10) and are approximately the same size as the pycnia. In some cases, aecia may be found on leaf veins on the underside of the leaf (Fig. 11). In some production regions, such as the northern and central U.S. Great Plains states, the pycnial and aecial stages commonly occur early in the growing season when conditions are favorable (Markell et al. 2009). In other production regions, this is a rare event. For example, the pycnia and aecia have never been observed on wild sunflower and rarely on commercial production in Australia; however, the race diversity identified from wild populations suggests that sexual recombination (and thus, the development of pycnia and aceia) can occur (Sendal et al. 2006). In all regions, aecia and pycnia are less commonly observed than either uredinia or telia.
| 
Figure 14. Color variations associated with infection by Puccinia helianthi (Sam Markell, NDSU).
| 
Figure 15. Dusty cinnamon-brown urediniospores of Puccinia helianthi on leaf and finger (Andrew Friskop, NDSU).
| 
Figure 16. Black telia of Puccinia helianthi (Sam Markell, NDSU).
| |
Uredinia are discrete, small (approximately 1 mm) pustules that may or may not be surrounded by a chlorotic halo (Figs. 12 and 13). While halos are generally chlorotic in commercial sunflower production, color variations may occur (Fig. 14). Uredinia contain dusty cinnamon-brown to rust-colored urediniospores (giving the disease its name), which for diagnostic purposes can easily be rubbed off host tissue, leaving a visible streak of spores on fingers (Fig. 15) (Markell et al. 2018). Uredinia can be observed at any time during the growing season but become increasingly abundant throughout the season, especially during an epidemic. The last visible stage is the telial, which produces small (approximately 1 mm) black pustules that are firmly attached to the leaf and cannot be rubbed off (Fig. 16). In severe epidemics, leaves will curl and dry, and uredinia and/or telia can be observed on petioles, stems, bracts, and heads (Figs. 17, 18, and 19).
Disease Cycle and Epidemiology
P. helianthi is present in all major commercial sunflower-producing continents and impacts all commercially produced (oilseed, confectionary, ornamental, birdseed, stockfeed, etc.) and wild sunflower (Gulya et al. 2016, Markell et al. 2015)
Helianthus spp. However, the impact on yield varies greatly by geography, climate, and time of infection (Gulya et al. 2019, Markell et al. 2015, Friskop and Markell 2016). The severity of an epidemic and level of yield loss is often influenced by the time or growth stage of epidemic onset, favorability of climate for disease development, and management tools utilized by growers (Friskop et al. 2011a, Friskop et al. 2011b, Friskop et al. 2015a, Harveson 2010a, Markell et al. 2009).
Disease epidemics can begin in two ways: 1) from overwintering locally dispersed sources of pathogen inoculum (commonly a result of completion of the sexual cycle); or 2) from long-distance airborne urediniospores from sources miles away. When epidemics begin from overwintering local sources of inoculum, early onset of the disease results in the increased potential for very high yield losses and are more likely to occur when commercial sunflower fields are planted near a source of overwintering inoculum from the previous year (e.g., a severely diseased field the previous year) (Friskop et al. 2011a, Markell et al. 2009). This commonly includes volunteer sunflowers from a previously infected crop or a stand of wild sunflowers known to have rust—both of which can act as an effective reservoir for the pathogen (Figs. 20 and 21). The presence of pycnia or aecia is evidence of successful overwintering of telia and subsequent completion of the sexual cycle; commonly, this will result in early onset of the disease (Friskop and Markell 2016, Gulya et al. 2019, Harveson 2010a, Markell et al. 2009). While aecia have been observed in as many as 60% of fields where rust occurs, it is more common that rust is not observed by growers until the uredinia stage (Markell et al. 2009, Gulya et al. 2019). In areas with mild winters, early-onset epidemics may also originate from locally overwintering urediniospores (Sendal et al. 2006).
When disease epidemics begin as a result of urediniospores dispersed long distances, pycnia and aecia will not be observed. Urediniospores from rust species are generally rugged and stable, allowing them to maintain viability and cause infection, even after traveling great distances. In North America, urediniospores of other
Puccinia species are known to travel in thewind from Mexico and south Texas north to the Dakotas and Canada along the
Puccinia pathway (Schumann and Leonard, 2000). Both commercial and wild sunflowers are abundant throughout the U.S. Great Plains states, and although evidence is lacking, distribution along the
Puccinia pathway may be possible. It may be more likely that the urediniospores infecting fields originate from nearby commercial fields or stands of wild sunflowers. When infection begins from a long-distance infection, time of onset may vary greatly, from vegetative stages to near maturity.
Under optimal environmental conditions, a repeating cycle of urediniospores may occur every 10–14 days at temperatures from 10 to 25oC, 24 h of free moisture, and low light intensity (Friskop et al. 2015a). However, infection will take place with far fewer hours of free moisture and a much wider range of temperatures (Markell et al. 2015) and is known to occur as rapidly as 6–7 days under ideal conditions (Sendal et al. 2006). Rust epidemics are more likely to occur in areas prone to frequent heavy dews and/or fog (Friskop et al. 2011a). Similarly, rust is commonly first established in areas where favorable microclimates exist, such as along tree rows or near buildings that may reduce wind, provide shade, lengthen dew periods, and reduce hours of direct sunlight. Dense canopies also create a favorable microclimate, particularly in the lower leaves that are usually the first plant part infected. Consequently, rust is commonly first observed (and is most severe) on protected leaves in the lower canopy (Friskop et al. 2011a). Even the microclimate on a single leaf will influence where rust occurs. It is common for a higher prevalence of uredinia to occur in areas of a leaf that have slightly longer prolonged periods of free moisture, such as along a leaf vein, in a leaf depression, or on the underside of a leaf (Figs. 22 and 23).
The geography and severity of epidemics of rust can be unpredictable. This is a result of the pathogen's ability to travel great distances, survive harsh conditions, and develop new races (that can overcome resistance genes). In Argentina, rust has historically been considered economically limiting in the northern sunflower-producing provinces of Chaco and Santa Fe, where it is common to see rust appear on early sunflower growth stages. However, in 2007, an epidemic occurred in the central- (south of Córdoba, La Pampa, north of Buenos Aires) and southern-producing (south of Buenos Aires) regions of Argentina (Huguet et al. 2008). The severe epidemic in 2007 was a result of 1) optimal environmental conditions for urediniospore dispersal and disease development; 2) abnormally early onset of disease; and 3) the emergence of more virulent race(s) of the pathogen. The latter a result of selection pressure applied to the pathogen population due to the utilization of a few resistance genes for long periods of time (30 years) (Huguet et al. 2008, Moreno et al. 2012).
Under ideal conditions, yield loss to rust can be extremely high and has been reported globally. Yield losses of approximately 80% were reported in the highest sunflower-producing state in the United States, North Dakota, in 2008 (Markell et al. 2009); see the “2008 Case Study–Sunflower Rust Epidemic Near Mohall, ND" section below for an in-depth look at rust epidemiology. Similarly, yield and oil content reductions of up to 70% have been reported in Argentina (Ivancovich et al. 1985).

Figure 17. Sunflower leaf with severe sunflower rust (Sam Markell, NDSU).
| 
Figure 18. Severe sunflower rust on sunflower stem and petiole (Sam Markell, NDSU).
|

Figure 19. Severe sunflower rust on sunflower bracts (Sam Markell, NDSU).
| 
Figure 20. Stands of
Puccinia helianthi-infected volunteer sunflowers along a roadside (left) adjacent to a commercial field (Sam Markell, NDSU).
|

Figure 21. Volunteer Helianthus spp. infected with Puccinia helianthi
| 
Figure 22. Sunflower leaf with high levels of uredinia along leaf veins, which tend to have longer free-moisture periods (Sam Markell, NDSU).
| 
Figure 23. High levels of
Puccinia helianthi uredinia are observed in depression of sunflower leaf (where free moisture collects), while the remainder of the leaf has very limited infection (Sam Markell, NDSU).
|
|
Disease Management
Resistant hybrids – Planting resistant hybrids is one of the most effective and environmentally friendly ways to manage rust on both oilseed and confection hybrids (Friskop and Markell 2016). However, with new races of the pathogen evolving, it is important to regularly scout fields even if a resistant hybrid is planted (Markell et al. 2016). Pathotyping urediniospores from isolated uredinia on new hybrids is a useful technique for identifying new races. Planting a range of hybrids with differing genetic backgrounds also helps minimize buildup of new pathotypes.
Managing wild sunflowers – If practical, elimination of wild and volunteer sunflowers may help manage rust. All known
Helianthus spp. can harbor this pathogen, and thus, it is important to manage both wild and volunteer sunflowers (Gulya et al. 1997). Early infections can lead to greater disease severity, more disease cycles within a growing season, and result in greater yield loss (Markell et al. 2009). Additionally, destroying undesired sunflowers can break the disease cycle and reduce the likelihood of the pathogen going through sexual recombination with the subsequent generation of new races (Friskop et al. 2011a).
Crop rotations – Crop rotations, including the destruction of diseased plant residues, can reduce inoculum and help break the disease cycle of
P. helianthi. This can also be an important management strategy for a number of other pathogens that infect sunflower, including
Sclerotinia sclerotiorum,
Plasmopara halstedii, and
Diaporthe helianthi (Gulya et al. 2019, Markell et al. 2015).
Foliar fungicides – Applying foliar fungicides in a timely manner can be an effective way to manage disease (Markell 2013). A number of fungicides have proven to be efficacious against rust, and action thresholds for rust management have been established (Friskop et al. 2015a). For example, research conducted on confectionary sunflowers in the U.S. northern Great Plains states determined a fungicide action threshold of 1% severity on the upper four fully expanded leaves occurring at or before growth stage R5 would limit yield loss (Friskop et al. 2015a). Frequent scouting of fields is important to determine if fungicide applications are warranted.
2008 Case Study – Sunflower Rust Epidemic Near Mohall, ND

|
Figure 24
|
In 2008, a widespread outbreak of sunflower rust occurred in the U.S. states of North Dakota and Minnesota (Markell et al. 2009, Gulya et al. 2019). The observations and management of the disease in a sunflower field near Mohall, ND, illustrates the epidemic potential and economic impact that sunflower rust can have when 1) a commercial field is planted next to a source of inoculum; 2) the pathogen successfully overwinters and completes its life cycle; 3) rust infection begins very early in the season; 4) environmental conditions are conducive for epidemic spread; and 5) when management tools are (and are not) used.
Pycnia and aecia were first observed on June 24, 2008, in a commercial confection sunflower field near Mohall, ND (Markell et al. 2009). Sunflowers were in early vegetative growth stages, and both pycnia and aecia were found on cotyledons and/or early emerged leaves. Prevalence was highest near a shelter belt (tree row) separating the field from an adjacent field planted to sunflowers the previous year. Ten days after pycnia and aecia were first found, the first uredinia were observed (July 4). By mid-July, while the field was still in vegetative growth stages, the lower leaves had rust severity levels between 10 and 20% (Fig. 24). In the majority of the sunflower field, a grower applied a fungicide to manage rust at two reproductive growth stages (early bud and early bloom). Fungicide applications (made available by an emergency exemption) were efficacious and applied at timings to slow or arrest the epidemic spread. At harvest, yield of the field was 1,571 kg/ha, nearly identical to the statewide yield average of 1,573 kg/ha in 2008, but believed to be slightly lower than the yield potential of the field. In a field strip where no fungicide was applied, mean disease severity on the upper four fully expanded leaves in the field was 56% on August 27, 2008. Harvested yield of the nontreated strips was approximately 200 kg/ha with unmarketable quality—a total loss.
Environmental conditions in this field were optimal for an epidemic to progress. Weather data collected in Mohall, ND, indicated that air temperatures would reach the dewpoint temperature in 51 of 61 nights in June and July 2008 (when air and dewpoint temperatures are the same, fog and/or dew frequently develop) (Markell et al. 2009). Thus, frequent periods of free moisture on leaves over the growing season provided ample opportunity for many urediniospore cycles (the "repeating stage").
This case study also demonstrates the epidemiologic and economic importance of use of management tools. It is likely that if this field had not been in such close proximity to a source of overwintering inoculum the initial infection would have begun later in the growing season. This would serve to decrease the number of repeating cycles and disease severity. The fungicide applications proved to be very effective, and in this case, an excellent economic investment. The cost of fungicides varies greatly (ranging from approximately a dollar per hectare for generic products to many dollars per hectare for premium fungicides), and there is a cost for an aerial application (fungicides are commonly applied by airplane). However, the cost of two applications is small compared to the value lost to the disease. If the grower had planted a rust-resistant hybrid, the epidemic may have never existed at all. However, rust-resistant hybrids are not always available, and even if one is, the decision to plant it is not without cost or risk. A premium price may be commanded for a new hybrid with desirable traits. Even if a premium price is not commanded for a suitable resistant hybrid, a potential indirect cost may exist if the grower must give up a different desirable trait (such as herbicide tolerance, insect resistance, etc.) to select a hybrid with resistance. Lastly, as
P. helianthi quickly overcomes resistance genes and develops new races, growers (and pathologists as well) may not know which gene(s) are likely to be most effective against the pathogen population (Markell et al. 2016, Gulya et al. 2019). The latter limitation demonstrates the importance of race surveys and close collaboration among pathologists and plant breeders when developing and recommending resistant hybrids. Further, the ability of the pathogen to overcome resistance genes demonstrates the importance of plant breeding strategies, such as gene pyramiding, to increase the durability of resistance that may prevent epidemics like the one near Mohall, ND, in 2008 (Meyer et al. 2020, Sendal et al. 2006).
Sunflower Rust Significance
Much of what we know today about the biology of rust fungi was first determined from the study of wheat stem rust. However, sunflower rust is also of great historical importance to the discipline of plant pathology. Its study has laid the foundation for our understanding of life cycles and the importance of sexual phases in all rust pathogens, begun first through the work of John Craigie (Craigie 1927, 1928).
John H. Craigie was a graduate student with the Dominion Rust Research Laboratory, Winnipeg, MB, Canada. In 1927, he discovered the functional role of the pycnial stage, which had been questioned for years (Craigie 1927). This brilliant idea was conceived after watching a fly landing and moving about on nectar-filled pycnial pustules of rust-infected, autoecious sunflower seedlings in the greenhouse. He mixed the exudate (nectar) emerging from the pycnia from several pustules and found that isolated infections arising from single pustules resulted in new pycnia with new pycniospores and nectar, but no aecia developed, as they often did when several pycnial pustules were positioned close on infected leaves. Craigie concluded that the spores must be different types that he referred to as (+) and (-), and when adjacent infections of single spores were of the opposite type, hyphal fusions then must occur, followed by the development of new aecia (Craigie 1927, 1928).
Sunflower rust has additionally served as a catalyst for further accelerating the knowledge base for this pathogen in plant pathology (Harveson 2015). Due to the severity of rust on sunflower production in the Soviet Union, it became the major target for breeding genetic resistance, one of the first examples utilizing this technique for managing a plant disease. Moreover, in the first decade of the 2000s, rust became severe enough in sunflower production in the United States that new fungicides were forced to be registered to save the entire industry (Harveson 2015).
Perhaps sunflower rust's most important contribution was serving as the model for deducing the purpose of the pycnial stage of rust fungi (Harveson 2018). It was not until 1927 that the functional role of the pycnial stage of rust pathogens in general was deduced by John Craigie. His landmark revelation was first demonstrated with the rust pathogen of sunflower, experimentally demonstrating that aecial formation occurred after transferring spores from one pycnium to another of the opposite mating type. This discovery proved that rust fungi had the ability to reproduce sexually, potentially creating new strains (races) of the fungi each season. This also explained the puzzling question of how resistant cultivars that yielded well one year would then inexplicably suffer huge losses from disease the next. It is also interesting to note that this observation first occurred from a disease and crop that were not economically important at the time of discovery (Harveson 2018, 2019).
