Aphanomyces root rot or common root rot of legumes
Aphanomyces euteiches
Weed and cultivated legumes; pea (Pisum sativum), alfalfa (Medicago sativa), snap bean and red kidney bean (Phaseolus vulgaris), faba bean (Vicia faba), red clover (Trifolium pratense), white clover (Trifolium repens), barrel medic (Medicago truncatula), lentil (Lens culinaris)

Pea roots showing honey-brown discoloration
associated with Aphanomyces root rot.
Symptoms and Signs
Aphanomyces root rot (ARR) is a disease that affects both annual and perennial crop species in the legume family. Symptoms of ARR are relatively common among both annual and perennial hosts, but timing and pattern of disease occurrence often differ. Because A. euteiches is a root-infecting pathogen, primary symptoms occur on roots and subterranean stem tissues. Initially, infected root tissue appears gray and water-soaked, becoming soft and honey-brown or blackish-brown in appearance (Figure 1). Eventually roots are reduced in volume and function (Figure 2). It is common for symptoms to advance from roots into the stems, which is often typified by chlorosis (yellowing) of the cotyledons and necrosis (death and discoloration) of epicotyls or hypocotyls (Figures 2, 3, 4). Primary symptoms of roots and stems will eventually lead to secondary symptoms of chlorosis, necrosis, and wilting of the foliage (Figures 2, 3, 5). Pre-emergence damping-off (sudden death/decay of seedling) is not commonly associated with ARR. Instead, seedlings are stunted (Figure 6) and less competitive against weeds (Figure 7). Roots infected by A. euteiches commonly have decreased nodulation, which contributes to symptoms of chlorosis. In the field, the pattern (locations) of symptomatic plants frequently corresponds to areas with poor soil drainage resulting from soils with a high clay content, compaction, and/or excessive irrigation

Figure 1 |

Figure 2 |

Figure 3 |

Figure 4 |

Figure 5 |

Figure 6 |

Figure 7 |
Many symptoms of ARR are common among hosts of A. euteiches while other symptoms are unique to an individual host. For pea and bean, lesions progress upwards on epicotyls and hypocotyls, respectively, and extend above the soil line (Figures 2, 3). Lesions on bean hypocotyls are water-soaked, gray-green in color and are firm. However, lesions on pea epicotyls eventually turn black and the tissues will collapse, resulting in a “pinched” appearance originating above the cotyledons. For alfalfa, hypocotyls seldom express noticeable symptoms, yet cotyledons are frequently chlorotic and will ultimately become necrotic (Figure 4).
Signs of A. euteiches are not visible to the unaided eye. Sexual reproductive structures, oogonia (female gametangia) and oospores (double walled spores resulting from sexual fertilization of a gametangium within the oogonium), can be readily seen in the cortical tissues of the root and hypocotyl with the aid of a compound microscope (Figure 8). One or more antheridia (male gametangia) may also be seen surrounding the oogonia, although they are ephemeral in the host tissue. Asexual reproductive structures, sporangia (specialized hyphae bearing asexual spores), primary spores, and zoospores (motile spores) of A. euteiches typically develop on, or near, the surface of host tissues (Figure 9). Formation of sporangia can be induced by placing pieces of infected root and hypocotyl tissues into a petri dish containing sterile water. After a 3- to 5-day period of incubation, sporangia bearing primary spores can be observed with either a stereoscope or compound microscope. Primary spores are spherical and are found loosely clustered, similar to a bunch of grapes, on the tips of a sporangium (Figure 9). The arrangement of primary spores on the sporangium is a characteristic feature of the genus Aphanomyces. Primary spores give rise to bi-flagellate (two flagella; whip-like structures that aid in locomotion) zoospores, which can be observed swimming in the water. Upon emergence, zoospores are attracted to roots in response to chemical signals released from roots.
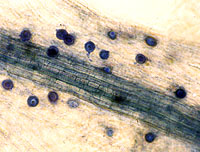
Figure 8 |
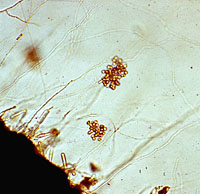
Figure 9 |
Pathogen Biology
Aphanomyces euteiches is classified as an Oomycete (a fungal-like protist), in the kingdom Chromista (Stramenopiles or Straminipila). Aphanomyces is in the order Saprolegniales, and is the order’s only genus to contain species pathogenic to plants. Aphanomyces cochlioides, a pathogen of sugar beet, and A. raphani, a pathogen of radish, are also economically significant pathogens within the genus. Like all Oomycetes, A. euteiches has coenocytic (no septa/cross walls) hyphae (Figure 10), cellulose in its cell wall (true fungi have chitin), and produces motile spores (zoospores). Nuclei are diploid (2N) in vegetative hyphae and all spore stages. It is a soil-borne pathogen that spends a part of its lifecycle in the soil and part of its lifecycle in its host.
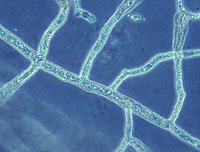
Figure 10
The sexual spore of A. euteiches, the oospore (Figure 11, 12), is a spherical structure with a double cell wall that remains dormant in the soil for several years in the absence of a host. Oospores are formed following meiosis. A haploid (1N) female gametic nucleus formed in the oogonium is fertilized by a second haploid nucleus (male gametic nucleus) that has migrated from an antheridium (male gametangium). Once fertilization occurs, a single oospore will develop within the oogonium. Aphanomyces euteiches is characterized as homothallic (same body) meaning both the oogonium and antheridium arise from the same hypha and are compatible (self-fertile). As a result, separate mating types are not required for sexual reproduction.
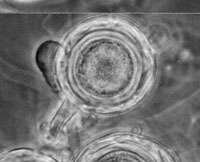
Figure 11 |
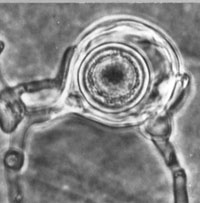
Figure 12 |
In the soil, oospores are stimulated to germinate by chemical signals exuded by host roots. Oospores form a germ tube that can either proliferate as hyphae or function as sporangia. Sporangia, which are indistinguishable from vegetative hyphae, arise from the germinating oospore and grow to about 8 to 10 times the diameter of the oospore. Nuclei (2N) migrate through the sporangium and develop a cell wall to form a primary spore. Spherical primary spores aggregate at the apex of the sporangium and will release zoospores that emerge through a pore in the cell wall of the primary spore. Zoospores are motile, kidney-bean shaped spores possessing one, long “tinsel” flagellum and one, short “whiplash” flagellum, both attached laterally. Both flagella assist the zoospore in migrating through water-filled soil pores towards host roots. Once a zoospore has reached the rhizoplane (root surface) it loses both flagella, encysts, and germinates by formation of a germ tube. Hyphae, derived from the germ tube, can directly penetrate epidermal host cells and colonize throughout root and subterranean stem tissues. Within host tissues, hyphae differentiate into antheridia and oogonia when host tissues begin to decline and oospores are formed.
The host range of A. euteiches includes several legume species. Within populations of A. euteiches, isolates express a diversity of pathogenic types some of which are host specific. The classification of isolates of A. euteiches for host specificity is based on the ability of the pathogen to progress into hypocotyls or epicotyls and initiate symptom development.
Currently, the forma specialis (f. sp.) concept (a taxonomic classification based on host range) has been applied to isolates pathogenic to pea, A. euteiches f. sp. pisi, and isolates pathogenic to bean, A. euteiches f. sp. phaseoli. This classification system is based on observations that A. euteiches isolates obtained from pea are non-pathogenic or weakly pathogenic on bean and alfalfa, and isolates from bean are non-pathogenic or weakly pathogenic on pea and alfalfa (Figure 13). However, in vitro studies of isolates of A. euteiches from pea and bean indicate that they are sexually compatible and capable of exchanging genes for virulence. The forma specialis concept has not been applied to isolates of A. euteiches pathogenic to alfalfa or clover species although isolates from clover are weakly pathogenic on alfalfa, and vice versa (Table 1; Figures 14, 15).

Figure 13 |

Figure 14 |

Figure 15 |
Table 1. Symptoms of Aphanomyces root rot (ARR) observed on root and stem tissue of various legume hosts by isolates of Aphanomyces euteiches (Ae).
| |
Pea |
Bean |
Alfalfa |
| Origin of Ae isolate |
Root |
Epicotyl |
Root |
Hypocotyl |
Root |
Hypocotyl |
| Pea |
+a |
+ |
+ |
-b |
+ |
+ |
| Bean |
- |
- |
+ |
+ |
+ |
- |
| Alfalfa Race 1 |
+ |
+ |
+ |
- |
+ |
+ |
| Alfalfa Race 2 |
- |
- |
+ |
- |
+ |
+ |
a (+)-Isolation of Ae and symptoms of ARR observed in root or stem tissue.
b (-)-Isolation of Ae and symptoms of ARR were not observed in root or stem tissue.
As mentioned above, many isolates of A. euteiches are capable of infecting roots of several legume species. However, progression of the pathogen into the hypocotyl or epicotyl and the degree of pathogenicity vary by subpopulation of A. euteiches and the host species from which an isolate of A. euteiches was isolated. This suggests the host range of A. euteiches, as determined through controlled inoculations in laboratories and in greenhouses, may not represent the actual host range of A. euteiches in a natural environment.
Within a host species, cultivars may react differently to isolates of A. euteiches, suggesting the existence of races. The existence of physiological races has been reported for pea, but is most evident for isolates within the alfalfa population of A. euteiches. Currently, alfalfa isolates are categorized as either race 1 or race 2 based on differential responses of specific alfalfa populations to isolates pathogenic to alfalfa.
Disease Cycle and Epidemiology
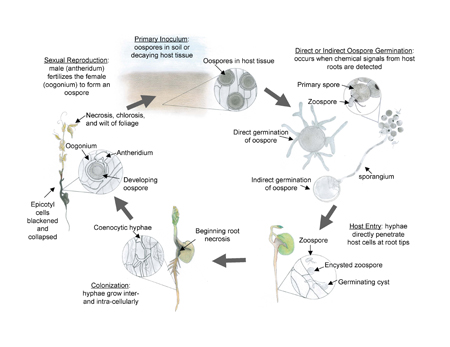
Disease Cycle
Epidemiology
Infection by A. euteiches can occur at any time during the growing season although it frequently occurs during the early phases of seedling emergence. The symptoms of ARR are sometimes difficult to distinguish from those caused by other root-infecting organisms such as Pythium, Rhizoctonia, and Fusarium. However, unlike Pythium, A. euteiches seldom causes seed rot or pre-emergence damping-off. The lesions caused by Rhizoctonia are sunken and cankerous whereas infection by Fusarium is distinguishable by the black or reddish discoloration of the vascular tissues, which can be readily observed in cross-section.
ARR is considered a monocyclic disease, which means only one infection cycle per season. Oospores within decaying host residue or in the soil germinate when chemical signals from host roots are detected. Germination can occur directly as a germ tube that ramifies by the formation of hyphae, or indirectly through the production of sporangia, which release primary spores and zoospores. Whether hyphae are derived from an oospore or from an encysted zoospore, infection can occur at any stage during the host’s development and within a large range of temperatures. However, infection and disease development are exacerbated when seedlings are infected and temperatures are optimal for host growth (22-28° C). Zoospores require water for movement to host roots. Standing water also predisposes host tissue to infection. Although water-saturated soils favor infection, symptoms and effects on yield are greatest if warm and dry soil conditions occur post-infection. Under optimal conditions, symptoms of disease are observed within 10 days following infection. Oospores form within necrotic tissues between 7 and 14 days. Oospores will either remain in host tissue or be released into the soil as roots decompose. Once formed, oospores remain dormant and can persist in the soil for more than 10 years, serving as primary inoculum when production of a host crop is resumed.
Disease Management
The most effective ways of managing ARR are through the use of resistant cultivars (Figure 5, 16), management practices that improve soil drainage, and avoidance of highly infested fields. Resistance has been identified in alfalfa, bean, pea and red clover and breeding for resistance to ARR has been most successful for alfalfa and bean. Until recently, combining horticultural and consumer-accepted traits with host resistance in pea has been problematic. In 2012, eight pea germplasm lines with acceptable agronomic characteristics and partial resistance to ARR were released for use in fresh, frozen, and dry pea production.
Crop rotation has been used to slow the rate of inoculum build-up in pea production areas. However, this practice is ineffective in reducing high inoculum levels due to the longevity of oospores. Once a field is determined to have a high ARR potential, growers are advised against planting a susceptible host. To assess ARR potential in a given field, soil samples are collected yearly and evaluated in greenhouse trials (Figure 17) following protocols originally developed at the University of Wisconsin-Madison in 1958. Other control strategies for ARR include preventing or correcting saturated soil conditions and compaction.

Figure 16 |

Figure 17 |
Fungicides, soil fumigants and biological control options, have been explored but have proven ineffective for use in commercial production. Additionally, A. euteiches is insensitive to metalaxyl and mefenoxam, two chemicals effective against many oomycetes such as Pythium and Phytophthora. The non-target effects of herbicides were studied in the 1970s as a means to lower the severity of ARR of pea. Dinitroaniline herbicides were shown to be detrimental to A. euteiches and were used to lower the severity of ARR in commercial pea production. Pendimethalin (i.e. Prowel®) and trifluralin (Treflan®) are active ingredients of the dinitroaniline herbicides that have had a suppressive effect on ARR of pea.
Cover crops and green manure crops have been studied as means to lower the severity of ARR of pea. Although using oats as a green manure has been successful, cruciferous plants have shown the greatest potential as an economical means of controlling of ARR. Isothiocyanates, thiocyanate ions, nitriles and epithionitriles are released from Brassica leaf or seed tissues by hydrolytic enzymes. These volatile compounds have detrimental effects on numerous microbes including A. euteiches. Although less feasible for large commercial acreages, the use of crucifer species as green manure crops has practical application for fresh market and organic growers who typically produce on a smaller scale.
Differences in soil nutrient content have been shown to influence ARR. In pea production fields, both the incidence and severity of ARR were negatively correlated with the concentration of calcium in the soil. In greenhouse studies, the application of calcium carbonate or calcium sulfate (gypsum) to soil with a high ARR potential significantly reduced disease severity.
Significance
Aphanomyces euteiches was identified in 1925 as the causal agent of a root rot disease that had plagued peas in Wisconsin for decades. Since its discovery, A. euteiches has been found throughout the U.S.A, Europe, Australia, New Zealand, and Japan. Initially, only pea was identified as a host of economic significance and ARR is still the most devastating disease of pea worldwide. On average, yearly losses to ARR are 10% although entire fields may succumb to the disease when environmental conditions are favorable for disease development. In the Great Lakes region of the U.S.A., pea production fields are intensively monitored for ARR potential and entire fields may be abandoned when inoculum levels reach a high ARR disease potential. Due to the longevity of oospores, fields with high ARR potential commonly remain unfit for pea production despite the absence of peas for years. The pea processing industry is typically located close to production fields to decrease the amount of time between harvest and processing. When fields are abandoned due to ARR, processing plants are closed and rebuilt near new production fields, often displacing workers and/or impacting local economies. The widespread occurrence of ARR in the Midwest has resulted in the pea processing industry decreasing pea production in the Midwest and eastern U.S.A. and increasing production in Idaho, Oregon and Washington where drier environmental conditions prevail. However, A. euteiches is common in the Pacific Northwest and can still cause significant yield losses in the region.
Many plant species were reported as hosts of A. euteiches prior to 1980. With the exception of pea, all were considered laboratory hosts and of no field relevance. A root disease of unknown etiology was observed on snap bean in Wisconsin in the 1970s. Symptoms were similar to several other root diseases, but different enough to indicate an unknown pathogen was present. Furthermore, methods of isolation from symptomatic tissues were inappropriate and delayed the identification of A. euteiches as the causal agent by Koch’s postulates until 1984.
Host specific forms of A. euteiches were determined to cause a root disease of alfalfa. For decades, the dogma was to avoid growing alfalfa in soils with poor drainage. However, the validity of this advice came to be seen in a new light with the discovery of Phytophthora medicaginis, the cause of Phytophthora root rot (PRR). PRR-resistant varieties were released in the early 1980s and alfalfa production was promoted for soils with drainage problems. Although PRR-resistant varieties provided a solution to unsatisfactory seedling establishment in many fields, poor seedling health and stand establishment remained an issue. By the mid 1980s, A. euteiches was confirmed as a significant pathogen of alfalfa, especially in conjunction with P. medicaginis. Since the initial studies in Wisconsin, A. euteiches has been identified as an economically important pathogen of alfalfa in other states. In Iowa, Kentucky, and Wisconsin, prevalence of A. euteiches in alfalfa fields often exceeds that of P. medicaginis. This observation may be partially explained by molecular studies of the interaction between A. euteiches and P. medicaginis within alfalfa roots, which suggest colonization by A. euteiches may reduce the colonization efficacy of P. medicaginis. Today, all modern alfalfa varieties are required to have resistance to both ARR and PRR to allow for successful seedling establishment and sustained forage yield.
Beginning in the early 1990s, variation in virulence was observed within populations of A. euteiches isolated from alfalfa. Isolates of A. euteiches virulent on alfalfa cultivars susceptible to ARR but avirulent on alfalfa cultivar WAPH-1, the hitherto source of resistance to ARR, were designated race 1. Isolates virulent to both susceptible alfalfa cultivars and race-1-resistant WAPH-1 were designated race 2. Race 2 isolates of A. euteiches are now prevalent in many alfalfa-producing states. Resistance to race 2 has been identified, which provides a yield advantage over ARR-susceptible cultivars and race 1-resistant WAPH-1, and race 2 resistant cultivars are now available to growers.
Selected References
Grau, C.R. 1990. Aphanomyces Root Rot. Pages 10-11 In: Compendium of Alfalfa Diseases. 2nd Ed. D.L. Stuteville and D.C. Erwin, eds. APS Press, St. Paul, MN.
Grau, C.R., A.M. Muehlchen, J.E. Tofte, and R.R. Smith. 1991. Variability in virulence of Aphanomyces euteiches. Plant Disease 75:1153-1156.
Heyman, F., Lindahl, B., Persson, L., Wikstrom, M., and Stenlid, J. 2007. Calcium concentrations of soil affect suppressiveness against Aphanomyces root rot of pea. Soil Biology and Biochemistry 39:2222-2229.
Holub, E.B., C.R. Grau, and J.L. Parke. 1990. Evaluation of the forma specialis concept in Aphanomyces euteiches. Mycological Research 95:147-157.
Malvick, D.K., and C.R. Grau. 2001. Characteristics and frequency of Aphanomyces euteiches races 1 and 2 associated with alfalfa in the midwestern United States. Plant Disease 85: 740-744.
Malvick, D.K., Grunwald, N.J., and Dyer, A.T. 2009. Population structure, races, and host range of Aphanomyces euteiches from alfalfa production fields in the central USA. European Journal of Plant Pathology 123:171-182.
McGee, R.J., Coyne, C.J., Pilet-Nayel, M.L., Moussart, A., Tivoli, B., Baranger, A., Hamon, C., Vandemark, G.J, and McPhee, K. 2012. Registration of pea germplasm lines partially resistant to Aphanomyces root rot for breeding fresh or freezer pea and dry pea types. Journal of Plant Registrations. 6:203-207.
Malvick, D.K. and J.A. Percich. 1998. Genotypic and pathogenic diversity among pea-infecting strains of Aphanomyces euteiches from the central and western United States. Phytopathology 88:915-921.
Muehlchen, A.M., R.E. Rand, and J.L. Parke. 1990. Evaluation of crucifer green manures for controlling Aphanomyces root rot of peas. Plant Disease 74:651-654.
Munkvold, G.P., W.M. Carlton, E.C. Brummer, J.R. Meyer, D.J. Undersander, and C.R. Grau. 2001. Virulence of Aphanomyces euteiches isolates from Iowa and Wisconsin and benefits of resistance to A. euteiches in alfalfa cultivars. Plant Disease 85: 328-333.
Pfender, W.F., D.K. Malvick, F.L. Pfleger, and C.R. Grau. 2001. Aphanomyces Root Rot. Pages 9-13 In: Compendium of Pea Diseases and Pests. 2nd Ed. J.M. Kraft, and F.L. Pfleger, eds. APS Press, St. Paul, MN.
Pfender, W.F., and L.E. Hanson. 2005. Aphanomyces Root and Hypocotyl Rot. Pages 10-12 In: Compendium of Bean Diseases. 2nd Ed. H.F. Schwartz, J.R. Steadman, R. Hall, and R.L. Forster, eds. APS Press, St. Paul, MN.
Shang, H., C.R. Grau, and R.D. Peters. 2000. Evidence of gene flow between pea and bean pathotypes of Aphanomyces euteiches. Canadian Journal of Plant Pathology 22:265-275.
Sherwood, R.T., and D.J. Hagedorn. 1958. Determining common root rot potential of pea fields. University of Wisconsin-Madison Agricultural Experimental Station Bulletin 531.
Smolinska, U., M.J. Morra, G.R. Knudsen, and P.D. Brown. 1997. Toxicity of glucosinolate degradation products from Brassica napus seed meal towards Aphanomyces euteiches f. sp. pisi. Phytopathology 87:77-82.
Teasdale, J.R., R.G. Harvey, and D.J. Hagedorn. 1978. Suppression of pea (Pisum sativum) root rot caused by Aphanomyces euteiches by dinitroaniline herbicides. Weed Science. 26:609-613.
Vandemark, G.J., Ariss, J.J., and Hughes, T.J. 2010. Real-time PCR suggests that Aphanomyces euteiches is associated with reduced amounts of Phytophthora medicaginis in alfalfa that is co-infected with both pathogens. Journal of Phytopathology 158:117-124.
Vincelli, P., J. Henning, T. Hendrick, J. Brown, L.J. Osborne, B. Prewitt, V. Shields, D. Sorrell, K.D. Strohmeier, R. Tackett, and J.W. Wyles. 2000. Improved seedling health, yield, and stand persistence with alfalfa resistant to Aphanomyces root rot. Agronomy Journal 92: 1071-1076.
Wiersma, D.W., C.R. Grau, and D.J. Undersander. 1995. Alfalfa cultivar performance with differing levels of resistance to Phytophthora and Aphanomyces root rots. Journal of Production Agriculture 8:259-264.
