Armillaria root disease, shoestring root rot
Armillaria species
Many woody angiosperms (hardwoods) and gymnosperms (conifers) in native forests, planted forests, orchards, vineyards, and in amenity plantings in urban areas. Some nonwoody plants are also hosts

Armillaria gallica mushrooms on an old stump, New York, U.S.
Symptoms and signs
Armillaria root disease is usually easy to diagnose conclusively if one can cut into the tree roots or base. A pulaski (Figure 2) is a good tool for diagnosis of root diseases of trees, because it has a digging/scraping side and a chopping side.

Figure 2
Crown symptoms
Branch dieback and crown thinning are common symptoms of Armillaria root disease, but they are also symptoms of other root diseases and other tree problems. On some conifers, chlorosis (yellowing) or reddening of foliage or heavier-than-normal production of cones (a stress crop of cones) may also appear.
Basal symptoms
On some species, the fungus may grow up from the roots in the inner bark and cause basal cankers above the infected roots. On resinous conifers, resinosis (Figure 3), an exudation of resin, accompanies and can occur proximal to infection of roots, the root collar and the stem base. When resinosis occurs, it is the most diagnostic of symptoms for Armillaria root disease. In some cases, decayed roots may be apparent or decay in the inner wood of the stem base (butt rot; Figure 4) may be exposed.

Figure 3 |

Figure 4 |
Armillaria species cause a white rot of wood (Figure 5), as opposed to brown rot. In white rot, both lignin and polysaccharides (cellulose, hemicelluloses) are ultimately degraded. Wood often has a bleached, whitish appearance because the brownish lignin is removed. During decay, there is undecayed, intact cellulose present that gives the wood a fibrous texture; i.e., the orientation of the grain is always apparent. In brown rot, in contrast, cellulose and hemicellulose throughout the wood are depolymerized in early stages, and the lignin is left relatively unchanged. As a result, wood loses its fibrous structure in the early stages of brown rot, tends to shrink and crumble easily, and becomes browner. Wood decayed by Armillaria species is often spongy or stringy, and may be wet. Black lines called zone lines are usually seen in the decayed wood. These lines are actually curved planes in the wood, sometimes called pseudosclerotial plates, composed of thickened, dark fungal cells. They may play a role in protection of Armillaria from unfavorable conditions or other fungi that attempt to invade its territory, including other individuals of the same species. Actively decaying wood may be luminescent, producing a faint glow that is visible in the dark (see Significance).
Signs
Three signs of Armillaria root disease may be seen in the field. Any one of them, correctly identified, permits a conclusive diagnosis.
Mycelial fans (Figure 6) are nearly always present in infected and recently killed trees. These are white mats of fungal mycelium, between the inner bark and wood. Although other fungi may form mycelial fans, those of Armillaria species are generally more substantial, have a mushroom odor, often have a fan-like pattern, and may be peeled off in small pieces.

Figure 5. |

Figure 6. |
Rhizomorphs (Figures 7, 8) are commonly associated with infection. Depending on the species of Armillaria, they may be few, small, fragile and hard to find or abundant and robust. They are cylindrical (in soil) or flattened (under bark), 1-5 mm (0.04 - 0.20 in.) diameter, reddish-brown to black, branched, and have a cream-colored tip when actively growing. The inner tissue is whitish mycelium, but it may become tan with age. Rhizomorphs are often attached to infected roots, but they may also be attached to the surface of uninfected roots. Although other fungi produce mycelial cords and rhizomorphs, I am aware of no other fungus with rhizomorphs that would be confused with those of Armillaria species. They are much more likely to be confused with roots: even experienced workers sometimes have to examine them carefully to identify them as rhizomorphs!

Figure 7 |

Figure 8 |
Mushrooms (Figure 1) may be produced in late summer or autumn. Fruiting is erratic; mushrooms may be absent in some years and very abundant in others. Although mushroom morphology varies with the species, they are generally in clusters near or on the base of trees. Caps are honey-brown, usually with small tufts of dark hairs, gills are whitish with notched attachment, spore prints are white, and stems are white to brown, usually with an irregular, mottled appearance. Most species have a partial veil that results in a more or less delicate annulus (ring) on the stem. Other differences among species in mushroom morphology are often subtle and variable. The mushrooms are edible, although you should not eat wild mushrooms unless you are with an experienced collector

Figure 1 |
Pathogen Biology
Armillaria is in the phylum Basidiomycota. Basidiospores are produced in mushrooms, specifically on basidia that line the gills on the underside of the cap (see Symptoms and Signs for more information on mushrooms).
Armillaria species have an unusual nuclear cycle that is not yet completely understood. According to most studies, they differ from related members of Basidiomycota in that the mycelia, after mating, are predominantly diploid (2n) rather than heterokaryotic (different haploid nuclei sharing a mycelium).
Sexual Reproduction
Sexual compatibility among single-basidiospore isolates is regulated by two mating-type loci, each with multiple alleles in the population. Isolates must have different alleles at both loci to be sexually compatible. If they are, the hyphae can fuse, then nuclei fuse shortly afterwards to establish a diploid mycelium. Such a mycelium is the dominant phase that one finds growing in wood, growing through soil as rhizomorphs, and killing trees. Eventually, when conditions are suitable, mushrooms are produced. There may be, in some species, some intermediate nuclear changes, but ultimately, in special cells (basidia) lining the gills of the mushroom, meiosis results in the formation of four haploid nuclei. Each nucleus enters a developing basidiospore, completing the sexual cycle.
Asexual Reproduction
Armillaria species do not produce asexual spores, but they are able to disperse locally and colonize new trees. They do this either by growing as mycelium through root contacts or root grafts between two trees, or by growing through the soil as rhizomorphs (1-5 mm in diameter) to a nearby tree (Figure 7).

Figure 7 |
Species
Although many species of Armillaria had been described over the years, their characteristics and differences were uncertain. Plant pathologists recognized there was variability in the pathogen, but difficulty in resolving that variability resulted in lumping the pathogen under the species A. mellea. This changed after it was demonstrated that there were intersterile groups, or biological species, of Armillaria. Within a group, mating followed a normal mating system, but generally no mating occurred between the groups. Gradually, often subtle differences between the groups in host preference, aggressiveness, geography, mushroom morphology, and DNA were demonstrated. Most of the intersterility groups have now been linked to previously described species or have been described as new species.
Today the genus Armillaria is thought to contain about 40 species. Some occur in multiple continents and some are relatively restricted to a region of a continent. About nine species are recognized in North America. Two important pathogens, A. ostoyae (usually on conifers) and A. mellea (in the strict sense; usually on angiosperms) are probably circumboreal in distribution, meaning they occur around the northern hemisphere.
Disease Cycle and Epidemiology

Figure 9. Disease Cycle |
Epidemiology
Armillaria species have two means of dispersal and there are two corresponding parts of the disease cycle (Figure 9; click on image to see animation). The first means of dispersal is by airborne, sexual basidiospores, and this may occasionally result in establishment of a new infection center. The second means of dispersal is growth of the pathogen from tree to neighboring tree, either as mycelium that is transferred where diseased roots contact one another, or as rhizomorphs that grow through soil until they contact and infect a nearby, susceptible host.
Role of basidiospores
The role of basidiospores has long been an enigma in the disease cycle. We know that Armillaria species commonly produce mushrooms and large numbers of spores, but attempts to demonstrate infection or saprobic establishment by basidiospores in nature have usually been unsuccessful. However, there is abundant, if mostly indirect, evidence from studies of population structure and occurrence of the disease in plantations on previously unforested sites, indicating that establishment of the fungus from basidiospores does happen on occasion. Variation in population structure suggests that variation in the frequency of this occurrence may be due to climate, especially the moisture regime.
Local transmission and disease centers
Local dispersal by fungal growth is certainly the most common source of infection. It occurs by transfer of mycelium at root contacts and grafts or by growth of rhizomorphs through soil to nearby trees. The source of inoculum may be a root system of a tree recently infected or killed many years before. If the disease progresses this way, an expanding group of dead trees, often called a root-disease center, can develop (Figures 10-12). Typically the oldest mortality is in the interior and recent mortality and symptomatic trees are at the edge. Isolates from trees within a root disease center typically belong to the same genet, or clone, of the fungus. Although infection may not be spatially and temporally organized in this way, it is important to recognize disease centers when they occur to make a full diagnosis and understand the local epidemiology of the disease.

Figure 10 |

Figure 11 |

Figure 12 |
Survival and inoculum
Mycelium in colonized roots and the rhizomorphs produced from them serve as the most common inoculum. In general, larger pieces of woody inocula are more likely to cause infection than smaller pieces. Armillaria species may survive for up to 50 years or more in stumps, depending on the climate, size of stump, and other factors.
Stress
In some cases, Armillaria root disease causes most damage on trees that are stressed by one or more abiotic or biotic factors. These may include drought, defoliation, other diseases and insects, soil compaction and other soil problems, and numerous other factors. The role of stress in the disease is sometimes generalized too much, and the disease is described as generally being secondary to some other problem. In fact, the disease is sometimes primary, attacking otherwise healthy hosts, and in other cases secondary, attacking stressed hosts.
An example of a secondary, stress-related disease is in oak (Quercus) forests of the eastern United States. Armillaria gallica is normally a pathogen in such forests, but it tends to cause butt rot (slow decay of the inner, older wood of major roots and the base of the stem) and rarely kills trees. It is also an efficient saprobe, colonizing root systems of dying trees and stumps. However, when trees are defoliated over several years by gypsy moth (Lymantria dispar, a nonnative insect), trees become more susceptible, the more vital tissues of the root and stem base are attacked by A. gallica, and trees are killed. Although the disease is stress-related and secondary here, it is the difference between life and death for these trees: those that are not attacked by Armillaria root disease can usually recover from defoliation and again grow normally in the absence of further defoliation.
In contrast, A. mellea causes a lethal, primary disease in many conifer forests and plantations, and in many orchards and vineyards. In some cases, inoculum for such disease has been traced to root systems of previous forest trees, but in some cases spread occurs among crop trees and vines also.
Disease Management
Although Armillaria root disease is among the most studied diseases of trees in the world, and there are many potential approaches to disease management, the fact remains that, in many cases, we do not have an effective, practical means of reducing the disease. Further, we do not understand and cannot confidently predict the impact of some management activities, such as silvicultural thinning, on the development of the disease. However, in some areas and in some host systems, some among the following approaches may be effective at reducing disease incidence.
In cases where the disease is determined to be secondary, following some other stress factor, management may be best directed at alleviating the stress rather than directly controlling Armillaria root disease.
Tree species selection
Although many tree species are hosts of Armillaria root disease, there is usually variation in susceptibility among local species. In some areas, disease severity is associated with a shift of species composition. For instance, in many forests of the western United States, shade-intolerant pines occur early in successional sequences, but are maintained by fire, which tends to kill the shade-tolerant Douglas-fir and true firs that grow among the pines. Reduction in fire and selective harvesting of pines since European settlement have led to a shift away from the resistant pines to more shade-tolerant, susceptible Douglas-fir and true firs. This unnatural situation is responsible for high levels of root disease in some areas.
When opportunities arise to influence species composition, relatively resistant species can be favored. This can occur during planting, regeneration cuts, thinning, and partial harvests. Local seed sources should be used to maximize resistance in the local environment.
In horticultural and amenity plantings, any flexibility in species can be exercised in favor of resistant species. Within many horticultural species, varieties are known that vary in resistance. Such varieties are often used as rootstocks.
Inoculum removal
 Robert Hartig, in 1874, first recommended removal of infested root systems from soil as a means of reducing future infections. Stump removal, at least in part for disease control, has been a common practice in high-value orchard crops for many years. More recently it has been found to be effective in forests. Although it is practiced in some forests, its practicality is limited to high-value sites, amenable slope and soil type, and certain silvicultural systems. It is most effective when combined with root raking to remove mid-sized roots that are not extracted with the stump.
Robert Hartig, in 1874, first recommended removal of infested root systems from soil as a means of reducing future infections. Stump removal, at least in part for disease control, has been a common practice in high-value orchard crops for many years. More recently it has been found to be effective in forests. Although it is practiced in some forests, its practicality is limited to high-value sites, amenable slope and soil type, and certain silvicultural systems. It is most effective when combined with root raking to remove mid-sized roots that are not extracted with the stump.
Avoidance
When there is a choice among sites for silvicultural or horticultural investment, the presence, species, and abundance of Armillaria should be determined in the candidate sites. Based on local knowledge of the behavior of Armillaria species, sites can then be chosen to minimize future disease. In addition to amount and species of Armillaria, other factors, such as soil or habitat type, may be known locally to be associated with hazard of Armillaria root disease and should be similarly considered.
An example of avoidance is establishment of new campgrounds in forests. Where Armillaria root disease is a potential concern, candidate sites should be compared in terms of tree species composition and the presence and abundance of Armillaria species. Considering this information in choosing a site would help in maintaining a desirable forest cover in the campground and avoiding problems with diseased trees that are hazardous to campers.
Thinning
Thinning is potentially a double-edged sword as it affects Armillaria root disease. Where tree density is causing stress, and stress is increasing disease, thinning should reduce disease. Similarly, thinning can be used to adjust species composition, to favor more resistant species in the stand. However, thinning may also stimulate disease by supplying stump resources to the fungus and increasing inoculum potential. We do not fully understand what determines which of these effects is dominant, so predicting the effects of thinning is difficult.
Biological Control
There is great promise and potential for use of biological control of Armillaria root disease. Most concepts target stumps, using antagonistic fungi to preemptively colonize or to eliminate Armillaria species in the wood. However, more research, much of it tailored to local hosts, conditions and fungal communities, is needed before it can become operational.
Soil barrier
In some situations, particularly in orchards, creating a barrier in the soil to root and rhizomorph growth may be a practical way to limit the pathogen's spread. Often called trenching, this is done by digging a trench down to 1 m (about 3 ft), lining it with plastic, and backfilling. To be effective, of course, the pathogen must be absent from the side of the trench to be protected.
Chemical treatment
Fumigants, such as chloropicrin, carbon disulphide, and methyl bromide are sometimes used in orchard crops to help eradicate inoculum from soil, usually after stump removal and before planting. Fumigants have also been used successfully to eradicate the fungus from stumps, but they are not used in practice. Soil treatments around existing trees, to prevent infections or cure existing infections, generally have been unsuccessful or inconsistent.
Significance of the disease
Armillaria root disease is often one of the most important diseases of trees in temperate regions of the world. The disease is important in native forests, planted forests, orchards, vineyards, and in amenity plantings in urban areas. The disease may also occur in nonwoody plants (Figure 13). Substantial disease losses occur in temperate portions of North America, Europe, Asia, Japan, southern Africa, Australia, and New Zealand and certainly elsewhere. The disease also occurs in some tropical areas, including tropical portions of Africa, South America, and Sri Lanka, but in many cases it is less serious than in temperate areas, or occurs primarily at high elevations.
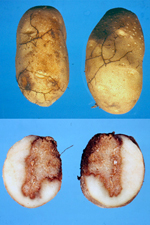
Figure 13 |
Humongous fungus
Small fields like mycology and forest pathology can be turned upside down when something strikes the media's fancy on a slow news day. Such was the case in 1992 when a scientific article appeared highlighting the ability of Armillaria gallica to form very large clones, or genets. All it took was for the media to link that concept with the phrase, 'humongous fungus', and the hounds were released! A front-page headline article in The New York Times, a feature on ABC News with Peter Jennings, and David Letterman's Top Ten followed in quick succession (Figure 14). Find more on this amusing story. Uhaul has a great web page on it, as it is one of the featured supergraphics on their rental vans. Even larger genets have been found. The largest to date is in eastern Oregon; it is about 900 hectares and estimated to be over 2,400 years old.

Figure 14
Luminescence
Perhaps the most wonderful thing about Armillaria is that the mycelium, especially in actively decaying wood, is bioluminescent. Such glowing wood is known commonly as foxfire. There is a reference to it in the oldest surviving piece of English literature, Beowulf. Find more on this glow-in-the-dark fungus here.
You can easily see the luminescence by finding a stump or dead tree colonized by Armillaria. Look for bright whitish decay that is active and not invaded by other fungi. Chop out pieces and put them in a plastic bag. Collect a quantity, as the luminescence varies among pieces. Now find a place as dark as you can. To get you and your friends in the proper frame of mind, look at it in the forest on a cloudy (dark and stormy?) night. First let your eyes adjust to the darkness by telling a spooky story or two. Now unveil your collections, feast your eyes and amaze your friends!
Selected References
Guillaumin, J.-J., C. Mohammed, N. Anselmi, R. Courtecuisse, S. C. Gregory, O. Holdenrieder, M. Intini, B. Lung, H. Marxmüller, D. Morrison, J. Rishbeth, A.J. Termorshuizen, A. Tirró, and B. Van Dam. 1993. Geographical distribution and ecology of the Armillaria species in western Europe. Eur. J. For. Path. 23: 321-341.
Harrington, T.C., J.J. Worrall and F.A. Baker. 1992. Armillaria. Pp. 81-85 in L.L. Singleton, J.D. Mihail and C.M. Rush, eds., Methods for Research in Soilborne Phytopathogenic Fungi. American Phytopathological Society Press. St. Paul, MN.
Hartig, R. 1874. Agaricus (Armillaria) melleus L. Der Hallimasch. Pp 12-42 and plates 1 and 2 In Wichtige Krankheiten der Waldbäume. Verlag von Julius Springer. Berlin, Germany.
Hartig, R. 1874. Important Diseases of Forest Trees. English translation by W. Merrill, D.H. Lambert and W. Liese, 1975. Phytopathological Classics No. 12. American Phytopathological Society. St. Paul, MN.
Lachance, D. 1996. Armillaria Root Rot. Information Leaflet LFC 14E, Natural Resources Canada, Canadian Forest Service, Quebec.
Shaw, C.G., III and G.A. Kile. 1991. Armillaria Root Disease. USDA Forest Service, Agricultural Handbook No. 691.
