Updated 2005.
Barley yellow dwarf
Barley yellow dwarf virus, Cereal yellow dwarf virus
barley, oats, wheat, maize, rice and other grasses

Like many plant diseases that are caused by viruses, barley yellow dwarf (BYD) is named for an economically important host (barley)
and the typical symptoms (yellowing, dwarfing) that develop when the host is infected with the virus.
Symptoms and Signs
The viruses that cause BYD infect over 150 species of cultivated, lawn, weed, pasture and range grasses. Some infected hosts display no obvious symptoms. However, in many hosts the most common symptom is stunting due to reduced internode length. The stunting may be so severe that heads fail to emerge or so mild that it is overlooked unless infected plants are carefully compared with uninfected plants. Root mass of infected plants is also often reduced. The most conspicuous symptom on infected hosts, loss of green color in leaves, is often more prominent on older leaves. Discoloration typically begins 1 to 3 weeks after infection and may be preceded by the development of water-soaked areas on the leaves (Figure 2). Barley leaves often turn bright yellow (Figure 3); oat leaves may become tan, orange, red, or purple (Figure 4); wheat leaves typically turn yellow or red (Figure 5); tips or edges of corn (maize) leaves turn red, purple or yellow (Figure 6); and rice leaves typically turn yellow or orange (Figure 7). Symptoms may be affected by the genotype, age and physiological condition of the host plant, as well as by the strain of the virus and the environmental conditions. Other symptoms of infection may include upright and stiff leaves and serrated leaf borders, reduced tillering and flowering, sterility and failure to fill kernels, which results in fewer and smaller kernels and corresponding yield losses.

Figure 2 |

Figure 3 |

Figure 4 |

Figure 5 |

Figure 6 |

Figure 7 |
Pathogen Biology
The viruses that cause BYD are typically 25-28nm in diameter and hexagonal in outline (Figure 8). They are composed of two proteins (a major coat protein and a minor "readthrough" protein) that encapsulate the single-stranded ribonucleic acid (ssRNA) genome. This RNA genome serves as a messenger RNA and has five to six genes or open reading frames (ORFs) (Figure 9). Some proteins are produced directly from the ORFs of the genomic RNA, while others are expressed from shorter RNAs, called subgenomic RNAs (sgRNAs).
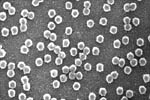
Figure 8 |
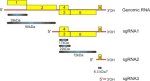
Figure 9 |
The viruses that cause BYD are restricted to the phloem of host plants, where they are seen via electron microscopy in the cytoplasm, nuclei and vacuoles of infected sieve elements, companion and parenchyma cells (Figure 10). Vesicles containing filaments and inclusions containing virus particles are common cytopathological effects of virus infection. The infection and subsequent death of phloem cells inhibits translocation, slows plant growth, and induces loss of chlorophyll, resulting in typical symptoms (see Symptoms).
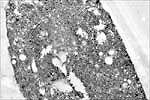
Figure 10 |
Disease Cycle and Epidemiology
Disease Cycle
The virus particle, or virion, is deposited in a phloem cell by an aphid vector. The virus replication cycle (Figure 11) begins when the ssRNA is released from the virion. This ssRNA is a positive sense or “+ sense” RNA that serves as a messenger RNA
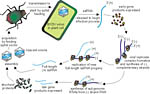
Figure 11 |
Early gene products (proteins) are translated from the ssRNA. These early gene products are believed to be part of the complex required to make copies of the viral RNA.
Complementary (negative sense or “- sense”) RNA strands are produced from the + sense ssRNA. The - sense RNA strands are then used as templates for the production of many copies of full-length + sense RNA. It is believed that these new + sense RNA strands are transported from cell to cell in the host, initiating more replication cycles and thus spreading the infection within the plant.
Subgenomic + sense RNAs also are made from the - sense RNA strands, and late gene products are expressed from these subgenomic RNAs. Among the late gene products are the structural proteins of the virions.
Full-length + sense RNA and structural proteins are assembled into virions, which can be ingested by an aphid vector that transmits the virus to an new cell, in the same or another plant, where the replication cycle can begin again. Newly formed virions also spread within the host plant through the phloem (Figure 12), but are not believed to initiate new infections within the plant.
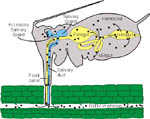
Figure 12 |
Epidemiology
The viruses that cause BYD are transmitted from plant to plant by at least 25 different species of aphids (Figure 13). The viruses cannot be transmitted mechanically (by rubbing), and there is no evidence that they multiply in their aphid vectors or are present in new-borne aphids. Thus, all aphids must acquire the viruses by feeding on infected plants. The viruses travel up the aphid’s stylet, through the food canal and into the gut, where they are transported into the aphid’s body cavity, or hemocoel (Figure 12). The viruses then circulate through the hemocoel to the aphid’s accessory salivary gland where they pass into the saliva and can be expelled into the phloem of another plant. The viruses cannot be transmitted by an aphid until they follow this path through the body of the insect, so there are usually several hours after an aphid acquires the viruses during which it cannot yet transmit them. This period of time is called the latent period.

Figure 13 |
This type of aphid transmission has been called “circulative” because the virus circulates in the body of the insect, or “persistent” because the aphid can retain the virus in its body for days or weeks. A single virus-carrying, or viruliferous, aphid can spread the virus to many plants as it moves and feeds. As nonwinged (apterous) aphids crawl to and feed on new plants in a field, small patches of infected plants will develop (e.g. Figure 7). Winged (alate) aphids (Figure 14) often develop as host plants begin to deteriorate or when the aphid population is overcrowded. Circulative transmission allows alate aphids to carry the viruses that cause BYD over long distances as they migrate, seeking new hosts. Thus, the sources of infection in a crop may be either local or at a great distance from the affected cereal field or grass pasture.

Figure 14 |
The different viruses that cause BYD are transmitted more efficiently by different species of aphids, a fact that was originally used to distinguish the viruses. For example, BYDV-MAV is efficiently transmitted by the aphid
Sitobion (formerly
Macrosiphum)
avenae. The acronym MAV came from
Macrosiphum avenae virus. Similarly, CYDV-RPV is most efficiently transmitted by the aphid
Rhopalosiphum padi. These differences in vector transmission are due to the fact that the viruses are selectively transported across the gut and salivary gland membranes of the aphids. For example, some of the viruses that cause BYD will be transported across these membranes in
R. padi, while others will not.
Environmental factors play several roles in the BYD disease cycle. High light intensity and relatively cool temperatures 15-18ºC (59-65ºF) generally favor expression of symptoms, such as leaf discoloration which may attract aphid vectors to virus-infected plants. The reproduction rate of subsequent aphid populations is affected by environmental conditions, as is the efficiency with which the aphids transmit the viruses. For example, transmission of the BYDV-RMV by the inefficient vectors
R. padi and
S. avenae is dramatically increased at high temperatures (30ºC/86ºF).
Disease Management
The first step in BYD management is accurate diagnosis of the disease. BYD symptoms may be confused with those caused by other biotic and abiotic factors. Therefore, visual diagnosis is unreliable, and diagnosis by laboratory techniques is required. The diagnostic test most commonly used to confirm infection is a serological test called enzyme-linked immunosorbent assay or ELISA (Figure 15).
Figure 15 |
Genetic resistance
As for many diseases caused by plant viruses, the most effective control strategies aim to prevent infection of host plants rather than to cure plants that are already infected. The method most widely used to reduce crop losses due to BYD is planting tolerant or resistant cultivars (Figure 16). In tolerant plants, viruses are still able to multiply, but few symptoms are expressed. In resistant plants, the ability of viruses to multiply and/or spread is impaired, resulting in a reduction of disease symptoms. Genes for at least partial tolerance or resistance have been identified in many of the crop plants infected by the viruses. Some plant species also have been genetically engineered to express portions of the viral genome, and some of these transgenic plants display high levels of tolerance to the disease.

Figure 16 |
Vector management
Another management strategy, which is sometimes feasible, is to reduce the introduction or spread of the causal viruses by aphid vectors. The viruses that cause BYD may be introduced into crops by aphids that migrate at similar times each year. Aphid populations can be monitored by catching the migrating aphids in traps (Figure 17); these aphids can then be tested for the presence of the viruses that cause BYD by ELISA or other assays.

Figure 17 |
When aphid migration patterns are known, it is sometimes possible to alter the planting date so that crops are older and more resistant when the seasonal migrations occur. Another strategy which may be applied when aphid populations are monitored is to reduce the spread of aphid vectors in crops through the use of insecticides. Insecticide treatments may not prevent initial virus infections, but can greatly limit the secondary spread of aphids and, therefore, of viruses. The economic costs and benefits of insecticides must be carefully calculated before an insecticide is used, particularly in relatively low value crops such as small grains. Finally, the introduction of predators and/or parasites of the aphid vectors as biological control agents has, in some cases, successfully reduced aphid populations and BYD spread.
Historical Significance
A century ago, oats were an important crop in the U.S. because they were "fuel" for the horses that were our principal means of transportation. In 1890 a mysterious disease of oats was seen from New England south to Georgia and west to Indiana and Illinois. The oats were stunted, sometimes turned red, tillered poorly, and often set little seed. Poor soil, lack of nutrients, and cold, wet weather were considered likely causes of this epidemic.
The next epidemic struck the U.S. oat crop in 1907. During this epidemic, an abundance of "plant lice," that we now call aphids, was noted on the affected oat plants. This led Ohio scientist T. F. Manns to study the possible relationship between aphids and the disease. Manns removed some aphids from diseased oats and caged them on healthy oats. He found that "Ten to twelve days after the caging the blight began to show, (while) the check outside the cage remained free (of disease)." Manns' observations and experiments led him to conclude that the aphids did indeed spread the disease, which he believed was caused by a bacterium.
Reports of red oats were common from the Pacific Northwest in 1918 and following years. Again, different abiotic (e.g. low temperatures, water-logged soils) and biotic (e.g. bacteria, fungi) agents were considered as possible causes. Most of these causes, however, could not explain why the red leaf symptoms persisted in spring oats from May through to maturity, or why symptomatic plants were found in a wide range of temperatures and soils.
It wasn't until 1951 that a virus was proposed as the cause of these epidemics in small grains. In April of that year, many barley fields in California turned brilliant yellow within a single week. By early May, severe yellowing symptoms were found in almost every barley-producing county of the state. Young barley plants were either killed or stunted so severely that they did not head. It was soon noticed that plants in nearby wheat fields were turning yellow (Figure 18), and that many nearby oats were turning red.

Figure 18 |
Because virus diseases do not usually affect crops so uniformly, a virus cause was probably not the first possibility considered. Again, the roles of climate, nutrition, fungi, and bacteria were examined. However, the presence of large aphid populations led Oswald and Houston to study the role of these insects in the disease. They found that five aphid species were capable of transmitting the disease. Oswald and Houston attributed the disease to a virus, but pointed out that this virus was not similar to most other plant viruses then known. The yellowing symptoms, transmission by aphids, and lack of transmission by rubbing (mechanical inoculation) differentiated it from other plant viruses. Barley yellow dwarf became one of the first and most widely studied of the "yellowing" virus diseases of plants.
Selected References
D'Arcy, C.J. and P.A. Burnett, eds. 1995. Barley Yellow Dwarf: 40 Years of Progress. American Phytopathological Society Press. St. Paul, MN
D'Arcy, C.J. and L.L. Domier. 2005. Luteoviridae. In Virus Taxonomy: VIIIth Report of the International Committee on Taxonomy of Viruses. eds. M.A. Mayo, J. Maniloff, U. Desselberger, L.A. Ball and Claude M. Fauquet. Academic Press. New York., NY.
Gray, S. and F.E. Gildow. 2003. Luteovirus-aphid interactions. Ann. Rev. Phytopathology. 41:539-566.
Miller W.A. and L. Rasochova. 1997. Barley yellow dwarf viruses. Annu. Rev. Phytopath. 35:167-190.
Oswald, J.W. and B.R. Houston. 1951. A new virus disease of cereals transmissible by aphids. Plant Disease Reptr. 35:471-475.
Plant Viruses Online. Descriptions and lists from the VIDE database.
http://www.image.fs.uidaho.edu/vide/genus026.htm
Smith, H.G. and H. Barker. 1999. The Luteoviridae. CABI Publishing. Wallingford, Oxon, U.K.
