Black knot
Apiosporina morbosa (Syn. Dibotryon morbosum, Plowrightia morbosum)
members of the genus, Prunus, mainly affecting cultivated plum, prune and cherry and to a lesser extent wild plum and cherry.

Black knot on plum twig (Used by permission of P.A. Arneson)
Symptoms and signs
The disease is characterized by the presence of thick, black, irregular swellings on the twigs (Figure 1). The presence of these symptoms is often first noticed in the winter season when they are unobscured by leaves. However, the fungal disease-causing agent has been present for quite some time. The pathogen's presence disrupts the normal growth of the twigs and a tumor-like growth forms at the infection site. Infections may take place as much as a year or more prior to the development of these characteristic "knots." Therefore, the swellings are normally not noticed until the winter of the second season of infection. It takes a keen observer to notice the subtle, initial symptoms present during the first season of infection.
The first symptoms appear as small, light brown swellings of the current or previous season's growth (Figure 2). By the next season the swellings turn olive-green in color with a velvety texture. Over this growing season the knots darken and appear to have a hard, brittle texture. The hard, black knots are the typical symptoms associated with the disease.
 Figure 1 Figure 1 |
 Figure 2 Figure 2 |
Knots vary in size from approximately 1-30 cm (0.5 to 12 in.) in length and from minute measurements to 5 cm (2 in.) in circumference (
Figure 3). The infected twigs often appear bent at the tips because of extra cellular growth on one side (
Figure 4).
 Figure 3 Figure 3 |
 Figure 4 Figure 4 |
Trees with heavy infections may contain numerous knots. Some of the older knots may appear white or pink in color. This discoloration is often seen in late summer and is caused by a fungal parasite, Trichothecium roseum (Figure 5).
 Figure 5 Figure 5 |
Pathogen biology
Black knot is caused by a fungus, Apiosporina morbosa (previously referred to as Dibotryon morbosum). The fungus belongs to the family Venturiaceae, which makes it closely related to the plant pathogen that causes apple scab, Venturia inaequalis. The fungus produces pseudothecia, fruiting structures that are embedded in the black stroma on the surface of the gall (Figure 6). The mass of pseudothecia is often referred to as the ascostroma. (Figure 7).
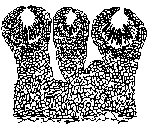 Figure 6 Figure 6 |
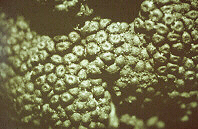 Figure 7 Figure 7 |
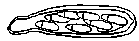
Figure 8 |
In the spring, two winters after initial infection, the fungus produces sexual spores called ascospores. The ascospores develop in asci (sacs) contained in the pseudothecia. Each clavate (club-shaped), bitunicate (double-walled) ascus contains eight ascospores. Each ascospore has two unequal-sized cells (Figure 8). The ascospores mature during the early spring of the infection's second season and are forcibly discharged into the air during rain events. The spores are distributed short distances on wind currents and through rain splashing.
The anamorph, or asexual, stage of the black knot fungus (Figure 8a) is a species of Fusicladium, that produces abundant olive-green conidia during the summer on the surfaces of one-year-old knots (Figure 9). It appears that the infection capabilities of the conidia are quite limited. Therefore, management strategies are focused on ascospore development and infection processes.
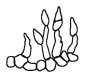 Figure 8a Figure 8a |
 Figure 9 Figure 9 |
Disease Cycle and Epidemiology
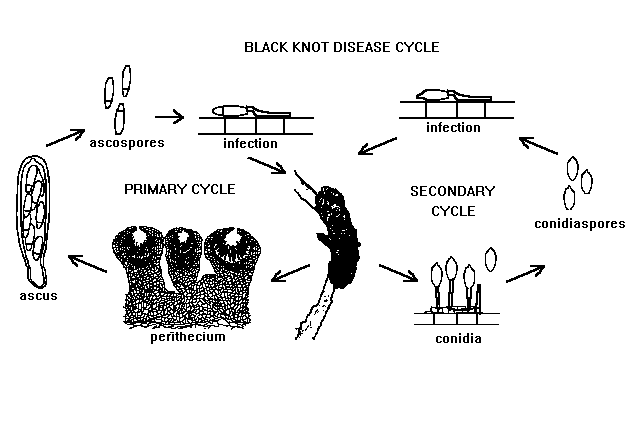
BLACK KNOT DISEASE CYCLE
Courtesy Wayne F. Wilcox
The fungus overwinters in the knots. About the time of bud emergence in the spring, the first ascospores are forcibly discharged from the ascostromata following a period of warm, wet weather. Apparently very short periods of wetness (only a few hours) are enough to prompt ascospore discharge. Temperatures between 16°C and 27°C (60-80°F) are ideal for the dissemination, germination, and infection of new plant tissue. Recent studies have confirmed and concluded that rainfall and temperature are the key factors in the release of spores and that the duration of the rainfall or wet period is not a factor.
The ascospores are spread by air currents and rain splashing. Mainly the succulent green shoots and, occasionally, wounded tissues are most susceptible to infection by ascospores. Ascospore discharge continues to occur 2-3 weeks after bloom. Infections take place during this time but may continue for a longer time period if susceptible host plant tissue is available. The germinating ascospores have the ability to penetrate unwounded surfaces of elongating, green shoots directly.
The knots develop very slowly, and by the end of the summer they appear only as small galls that might easily be overlooked (Figure 10). Further development does not occur until the following spring when the knots enlarge very rapidly. They initially are quite soft in texture and become greenish-brown in color as conidia develop over their surfaces (Figure 11). The conidia are disseminated by wind and splashing rain but probably do not figure as prominently as the ascospores in establishing new infections.
 Figure 10 Figure 10 |
 Figure 11 Figure 11 |
Ascospores are not produced until the spring following the second winter after initial infection. By the second summer after infection, the knots have enlarged considerably (
Figure 12) and begin to change in appearance to a hard, coal-black structure. The old knots enlarge every year by advancing at the margins (
Figure 13). The fungus mycelium can also spread internally and give rise to new galls some distance from the original knot. The central, older portions of the knot eventually break down and become riddled by boring insects (
Figure 14).
 Figure 12 Figure 12 |
 Figure 13 Figure 13 |

Figure 14 |
Disease Management
Cultural Management
Cultural management strategies are important in black knot management. Sites containing Prunus species should be monitored on a scheduled basis for possible infections. The main strategy to lower disease incidence is the removal of sources of inoculum. All shoots and branches bearing knots should be pruned out during the winter. This pruning should be completed before ascospore discharge begins in the spring, usually about the time that the buds first break. To be sure that even the unseen internal mycelium is removed, the cut should be made at least 15-20 cm (6-8 in.) below the knot (Figure 15). Winter is also a good time to look for and remove sources of inoculum in nearby wild Prunus species in hedgerows and woodlots (Figure 16). The knots are capable of producing ascospores for some time after removal from the tree. Therefore, they should be burned, buried, or removed from the site regardless of the time of year the pruning takes place.
 Figure 15 Figure 15 |
 Figure 16 Figure 16 |
Genetic resistance
When creating a new planting, consider selecting varieties with known resistance. Varieties may vary in their ability to tolerate or resist an infection.Black knot resistance is as important as fruit characteristics, tree size, and flowering time. Up-to-date listings of varieties with high levels of resistance are often available from local extension offices.
Site selection
Consider the site location. Avoid planting new trees near areas with known problems such as abandoned orchards or where wild varieties have been observed with the disease. When possible, remove wild varieties of the trees from the area.
Biological control
Interest in biocontrol agents is increasing because of the loss of certain fungicides registrations and the fact that applicators would prefer to reduce their exposure to pesticides. A possible biological control agent for black knot may be the fungal parasite, Trichothecium roseum, introduced in the Symptoms and Signs section.
Chemical Management
Fungicides are normally only recommended for sites with valuable trees and/or severe infections levels. Fungicides work best as a protectant and are ineffective if cultural practices are not also employed.
If a fungicide application is required, remember the biology of the organism. The applicator should pay particular attention to the weather conditions and inoculum levels when determining the timing and frequency of fungicide applications. Sites with severe black knot infections may require protective applications from early spring around bud break through summer (Figure 17). In some seasons the sprays can be terminated earlier if monitoring determines that inoculum is no longer available. Sites with low levels of inoculum may only need protection during the most susceptible period when ascospores are abundant and released in the spring. Regardless of frequency, fungicides have been found to be most effective when applied prior to a rain event and temperatures are above 16°C (60°F).
 Figure 17 Figure 17 |
Significance
Black knot is a disease that is most severe on cultivated crops in orchard and ornamental planting situations. Black knot appears to be a minor problem on Prunus species found in forest situations, where susceptible trees are surrounded by many nonsusceptible species of trees.
Black knot disease is mainly a problem in North America (Canada, the United States and Mexico) where it is indigenous. A record from 1979 indicated the presence of the fungus on pear in Taiwan but no other incidences have been reported from Asia. Currently it is not found in Europe or the EPPO region. The EPPO region consists of 43 European and Mediterranean countries that are responsible for international plant protection in their region. Apiosporina morbosa is listed as an EPPO A1 quarantine pest. Inclusion on the list requires the countries to follow phytosanitary regulations and to make appropriate requirements for admission of plant material in their countries.
Black knot has been reported on 24 species of Prunus but is most commonly found on wild and cultivated plums and cherries. Early publications from the 1950's describing the disease report no cases affecting peach, but a few rare infections have been reported since that time. The disease can be found throughout North America but is most commonly found in the northeast. It was first reported as a destructive disease in Massachusetts in 1811. It was first described in 1821 by L. D. Schweinitz from specimens collected in Pennsylvania. Researchers believe the disease is caused by a native pathogen that was only found in the northeastern states until around 1875, when observations of the disease began arising in the central states.
Young, infected twigs may die during the first year of infection. Larger branches may take several years to display severe damage. The infected trees decline and become more symptomatic with each growing season. The infection stresses the entire tree making it weaken, decline, and possibly die. The stress placed on the tree may also make it susceptible to infections by other pathogens.
Economically the trees lose value after a few years. The portions of a branch distal to a knot become stunted, and occasionally knots enlarge to girdle a branch and possibly kill it. Trees with multiple infections become dwarfed and misshapen, markedly reducing their productivity and attractiveness (Figure 18).
 Figure 18 Figure 18 |
Because of the long infection process and disease cycle, this disease is often overlooked by home gardeners and fruit producers. The leaves can mask the symptoms until firmly established infections are in place. Once established, it is very difficult to manage the disease. Commercial growers often discover the disease more quickly because they regularly inspect their trees during routine crop management. Awareness and strict monitoring of susceptible plants should be a priority for all home gardeners and commercial growers.
Selected References
Anderson, H. W. 1956. Diseases of Fruit Crops. McGraw-Hill Book Co., Inc., New York.
Apiosporina morbosa EPPO A1 quarantine pest factsheet. http://www.eppo.org/QUARANTINE/fungi/
Apiosporina_morbosa/DIBOMO_ds.pdf
Hickey, Kenneth D. 1966. Black Knot Disease of Plum and Its Control. Extension Publication Bulletin 1173, New York State College of Agriculture, Ithaca NY.
Horst, R. Kenneth. 2001. Westcott's Plant Disease Handbook, 6th ed. Kluwer Academic Publishers. Boston.
Jones, R.K. and D. Michael Benson. 2001. Disease of Woody Ornamentals and Trees in Nurseries. American Phytopathological Society, St. Paul, MN.
McFadden-Smith, W., J. Northover, and W. Sears. 2000. Dynamics of acospore release by Apiosporina morbosa from sour cherry black knots. Plant Dis. 84:45-48.
Ogawa, J. M. 1995. Compendium of Stone Fruit Diseases. American Phytopathological Society, St. Paul, MN.
Smith, D.H., F.H. Lewis, and S.H. Wainwright. 1970. Epidemiology of the black knot disease of plums. Phytopathology 60:1441-1444.
Ritchie, D. F., E.J. Klos, and K.S. Yoder. 1975. Epidemiology of black knot disease of 'Stanley' plums and its control with systemic fungicides. Plant Dis. Rptr. 59:499-503.
Wilcox, W. F. 1992. Black Knot of Plums. NYS IPM Fact Sheets for Tree Fruit. Cornell Cooperative Extension. Cornell University. Ithaca, NY.
