Black Sigatoka
Mycosphaerella fijiensis - found in nearly all the world's banana-growing regions.
Musa spp. (bananas and plantains)
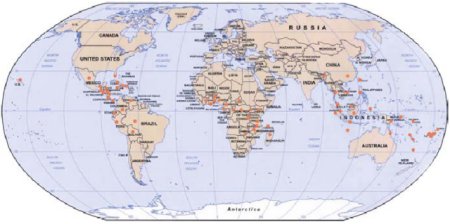
Figure 1. World distribution of black Sigatoka.

Figure 2. A banana plant.
Symptoms and signs
The first symptoms of black Sigatoka disease are tiny, chlorotic spots that appear on the bottom (abaxial) surface of the 3rd or 4th open leaf. The spots grow into thin brown streaks that are limited by leaf veins (Figure 3). The color of the streaks becomes darker, sometimes with a purple tinge, and visible on the top (adaxial) surface. The lesions then enlarge, becoming fusiform or elliptical, and darken to give the characteristic black streaking of the leaves (Figure 4). Adjacent tissue often has a water-soaked appearance, especially under conditions of high humidity.
 |
 |
| Figure 3 |
Figure 4 |
When the disease severity is high, large areas of the leaf may become blackened and water-soaked. On the necrotic tissue, numerous, tiny, black, globose fruiting bodies (pseudothecia) containing sac-like structures (asci) filled with ascospores will emerge from the underside of the leaf (Figure 5).
 |
| Figure 5 |
Black Sigatoka, first reported in Fiji in 1964, gets its name from appearing as a different form of yellow Sigatoka. It has black color in the young streaks and spots and lacks the distinct yellow halo that is present in young streaks of yellow Sigatoka. Although the lesions of yellow Sigatoka (Mycosphaerella musicola) may look similar, one can distinguish between the yellow and black Sigatokas by examining conidiophore structure (see table below). Mycosphaerella fijiensis produces conidiophores in small groups and not in the larger clusters (sporodochia) produced by M. musicola. Also M. fijiensis has basal scars on the conidium and conidiophore at their points of attachment. Mycosphaerella fijiensis produces most of its asexual spores (conidia) and structures producing male sexual spores (spermagonia) on the underside of the leaf, whereas M. musicola produces conidia predominantly on the upperside of the leaf. Symptoms of yellow Sigatoka (Figures 6 and 7) typically develop more slowly than symptoms of black Sigatoka (Figure 8) (See below: Yellow and Black Sigatoka Compared). In addition, diagnosis can also be accomplished through the use of polymerase chain reaction (PCR).
 |
 |
| Figure 6 |
Figure 7 |
 |
| Figure 8 |
If left unchecked, black Sigatoka will progress through the plant's leaf surface area, greatly reducing photosynthetic capability and thus yield (Figure 9).
 |
| Figure 9 |
Black and Yellow Sigatoka Compared
| Pathogen |
| YELLOW SIGATOKA |
BLACK SIGATOKA |
Mycosphaerella musicola
(Pseudocercospora musae) |
Mycosphaerella fijiensis
(Pseudocercospora fijiensis) |
- conidiophores formed in dense clusters (sporodochia) on dark stromata on both leaf surfaces
- conidiophores straight, usually nonseptate and unbranched, no spore scars
- conidia uniform thickness for full length, 1-5 septate, no distinct basal scar
|
- conidiophores formed singly or in small groups (2-5) on lower leaf surface
- conidiophores straight or bent, 0-3 septate and occasionally branched, slightly thickened spore scars
- conidia taper from base to apex, 1-6 septate, distinct basal scar
|
| Hosts |
| YELLOW SIGATOKA |
BLACK SIGATOKA |
| Bananas (AAA) generally susceptible; most cooking bananas and plantains (AAB and ABB) moderately to highly resistant |
Most dessert bananas, cooking bananas, and plantains susceptible |
| Symptoms |
| YELLOW SIGATOKA |
BLACK SIGATOKA |
- early streak pale yellow
- streaks appear on leaf numbers 4-5 (unsprayed Cavendish)
|
- early streak dark brown
- streaks appear on leaf numbers 2-4 (unsprayed Cavendish)
|
| Epidemiology |
| YELLOW SIGATOKA |
BLACK SIGATOKA |
- more common in cooler environments
- inoculum consists of both conidia (water-dispersed) and ascospores (wind-dispersed)
- conidia first appear in the mature spot stage
- produce more than 30,000 condia per spot
- conidia not dislodged by wind
- mature ascospores produced 4 weeks after the appearance of streaks
|
- more common in warmer environments
- windborne ascospores are the major inoculum
- conidia first appear in early streak stage
- produce about 1200 condia per spot
- conidia both water- and wind-dispersed
- mature ascospores produced 2 weeks after the appearance of streaks
|
Pathogen Biology
Sexual reproduction
Mycosphaerella fijiensis is the name given to the sexual form (teleomorph) of the pathogen. The fungus was first described in 1969 by Morelet on specimens from Fiji.
To produce the sexual form, the fungus first develops many spermagonia on the lower surface of the leaf as the lesions collapse. A spermagonium (Figure 10) is dark, somewhat erumpent, and pearlike in shape. In moist conditions these structures may ooze large quantities of the male reproductive cells (spermatia). Spermatia are tiny and cylindrical and will fertilize neighboring female receptive hyphae called trichogynes.
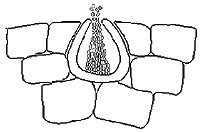 |
| Figure 10 |
Once fertilization is complete, pseudothecia are formed within the mature lesions, with their ostioles poking through the tissues (Figure 11). The oblong to club-shaped sac-like structures (asci) have two cell walls (bitunicate) and contain eight sexual spores (ascospores) that are lined up two-by-two. Pseudoparaphyses or sterile elements are absent from the pseudothecium. The ascospores are colorless and have one septum. One cell of the spore may be slightly broader than the other cell, and the spore may be slightly constricted at the septum. A squash mount of pseudothecia is shown in Figure 12.
 |
 |
| Figure 11 |
Figure 12 |
Asexual reproduction
The asexual form (anamorph) is called Pseudocercospora fijiensis. Conidia are borne on the conidiophore singly and terminally. The spores are pale to a light olive-brown, and are smooth, long, and have three or more septa (Figure 13).
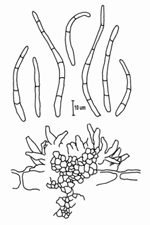 |
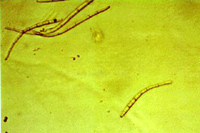 |
| Figure 13 |
Figure 14 |
Conidia germinate during periods of high relative humidity (92-100% relative humidity) and infect the leaf through a stoma, usually on the underside of a leaf. Under humid conditions, hyphae can emerge from the stomata, grow along the leaf surface and penetrate through other stomata, thus enlarging the lesions. Conidiophores emerge through the stomata, sometimes on erumpent, compact masses of mycelium (stromata). Stromata may also develop on young spermagonia.
Disease Cycle and Epidemiology
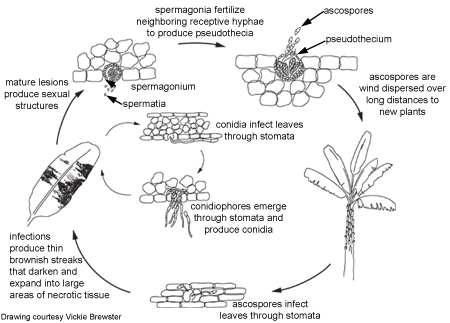 Disease Cycle
Disease CycleEpidemiology
With black Sigatoka, ascospores, and to a certain extent conidia, are the propagules by which the fungus is dispersed.
Conidia form readily in high humidity, especially if a film of free water is present on leaves. These asexual spores disperse during rain-wash and splashing, causing local spread of the disease.
Pseudothecia mature when dead leaf tissues are saturated with water for approximately 48 hours. Ascospores are the primary means of long distance dispersal and are the main means of spreading during extended periods of wet weather. Mycosphaerella fijiensis forms relatively few conidia, so ascospores are thought to be more important in the disease cycle.
Sigatoka leaf spot on bananas decreases somewhat during the dry season but otherwise produces more or less continuously repeated cycles of infection (Figure 15).
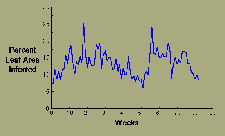 |
| Figure 15 |
Disease Management
Fungicides
Large plantations are heavily reliant on chemical controls. Control programs are largely based on the protectant fungicides mancozeb, usually applied in water or in combination with oil, and chlorothalonil. Mancozeb is often combined or rotated with morpholine, demethylation inhibitors (DMI), or strobilurin (QoI) fungicides. Cholorothalonil is rotated but not combined with other fungicides. Resistance to benzimidazole, DMI, and strobilurin fungicides is widespread in many production areas. The fungicides are often applied by airplanes (Figure 16).
 |
| Figure 16 |
Biological Control
Research into developing biological control methods for black Sigatoka has been limited since highly effective and affordable chemical controls are widely available for commercial growers. While biological control methods are desirable primarily for environmental reasons, their successful implementation is likely to be difficult since black Sigatoka is a polycyclic disease and susceptible banana tissue is present year-round. Various epiphytic bacteria (including Pseudomonas, Bacillus, and Serratia spp.) have been tested for controlling M. fijiensis, but biological control research is still in its preliminary stages.
Resistant cultivars
Use of resistant cultivars is really the only practical means of black Sigatoka control for the small-scale and subsistence farmer since fungicides are generally unaffordable. Unfortunately, while some resistant cultivars of plantains and bananas are available, they are often unacceptable to local tastes. Developing acceptable resistant cultivars is an important priority at international research centers (Figure 17).
 |
| Figure 17 |
However, breeding for disease resistance is particularly difficult with banana plants. Commercial bananas are autotriploids (AAA), i.e., they have three copies of the chromosome set instead of the two copies that the wild diploid species possess. While this extra chromosome set imparts favorable commercial characteristics such as seedlessness (unlike diploid wild species, Figure 18), as well as larger plant and fruit size, this high level of sterility is a major obstacle to banana breeders. Furthermore, the generation time (from seed to seed) for bananas is a long three years.
 |
| Figure 18 |
Cultural management
Cultural management techniques such as wider plant spacing, better drainage of both water and air, better weed management, and removal of severely diseased leaves or portions of them from plants can also be used to obtain some measure of control. Simply removing infected leaves (deleafing) and placing them on the ground can significantly reduce the efficacy of ascospore discharge (Figure 19). The application of urea and other products onto infested plant debris on the ground can accelerate decomposition and thus reduce further the available spore inoculum.
 |
| Figure 19 |
Quarantine and Sanitation
Proper quarantine and sanitation measures may provide some protection against two common means of long distance inoculum dispersal—leaves and rhizomes. Contaminated banana leaves are often used to protect the fruit when transporting bananas by truck.
Quarantine measures are practiced in some areas and countries where M. fijiensis has not yet been established or is confined to certain areas (Figures 20 and 21).
 |
 |
| Figure 20 |
Figure 21 |
Significance
Losses and Economic Impact
The black Sigatoka disease is particularly devastating. Under favorable conditions, leaf necrosis can reduce yields by 35-50%, and many important and commonly grown cultivars are susceptible. In 1995, the average cost for controlling this disease was US$1500/ha/yr. Annually, a typical plantation requires 38-50 fungicide sprays, and these sprays may account for around 30% of the production costs. In Central America, black Sigatoka may account for 27% of the total production cost, whereas other diseases and pests may account for only 3-5% of the total production cost.
Generally, a minimum of five leaves on the plant at harvest is required for fruit quality to remain stable during transportation. Fruit from severely diseased plants are prone to premature and uneven ripening. This is a grave concern for export producers, considering the exacting demands of consumers in developed countries.
While large-scale operations can afford to spray, most small growers cannot afford chemical controls and so suffer losses. This poses a serious food security concern to subsistence farmers, particularly for those who rely heavily on plantains in their diets. In certain parts of Africa, plantains are the primary staple food (Figure 22).
 |
| Figure 22 |
Selected References
International Mycosphaerella Genomics Consortium (IMGC)
Marín, D.H., R.A. Romero, M. Guzman, and T.B. Sutton. 2003. Black Sigatoka: an increasing threat to banana cultivation. Plant Dis. 87:208-222.
Ploetz, R.C., G.A. Zentmyer, W.T. Nishijima, K.G. Rohrbach, and H.D. Ohr. 1994. Compendium of Tropical Fruit Diseases. American Phytopathological Society Press. St. Paul, MN.
Ploetz, R.C. 1999. Black Sigatoka of Banana: The most important disease of a most important fruit. APSnet feature article: http://publish.apsnet.org/publications/apsnetfeatures/Pages/BlackSigatoka.aspx
Promusa Website:
http://www.promusa.org/
Stover, R.H. 1980. Sigatoka leaf spot diseases of bananas and plantains. Plant Dis. 64:750-756.
Stover, R.H. 1986. Disease management strategies and the survival of the banana industry. Ann. Rev. Phytopathol. 24:83-91.
Thurston, H.D. 1998. Tropical Plant Diseases. American Phytopathological Society Press. St. Paul, MN.
