Updated 2014.
Burrowing nematode disease; toppling disease, blackhead disease (banana); spreading decline (citrus); yellows, slow wilt (black pepper)
Radopholus similis
Over 350 plant hosts in tropical and subtropical regions including banana, citrus, black pepper, aroids (anthurium, philodendron, taro), ginger, tea, coconut and other tropical palms
Author
Fred E. Brooks
University of Hawaii at Manoa
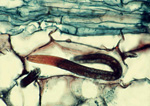
A burrowing nematode (Radopholus similis) feeds in the cortex of a ginger root. (Image by M. McClure, Univ. of Arizona, Bugwood.org)
Symptoms and Signs
Most soilborne plant pests and diseases are not evident aboveground until they are well established. Early symptoms caused by root feeding pests are due to impaired water and nutrient uptake. These symptoms include stunted plant growth, decreased vigor and yield, premature leaf drop and an increased tendency to wilt or dieback during dry periods. Radopholus similis causes a slow decline of many plant species, but symptoms are distinctive in banana and plantain (Musa hybrids and cultivars.), citrus (Citrus spp.) and black pepper (Piper nigrum)
Banana
The most dramatic disease symptom in banana plantations is the uprooting (toppling) of plants (Figure 2). Burrowing nematode feeding destroys anchor roots (Figure 3) and makes plants susceptible to toppling, especially when fruiting or during strong winds. Additional aboveground symptoms in bananas and plantains caused by root damage include slow sucker formation, delayed fruiting, smaller fruit and reduced bunch weight, and a shortened plant life. Radopholus similis kills feeder roots and creates reddish-brown lesions on larger root surfaces, both at the point of entry and throughout the cortex (Figure 4). Nematodes do not invade the central cylinder (stele), but heavy infestations can girdle and destroy roots (Figure 5). Eventually, burrowing nematodes can migrate from roots into the rhizome causing black, circular lesions, hence the name blackhead disease (Figure 6). Emerging roots may be attacked as they grow out of the infected rhizome.

Figure 2 |

Figure 3 |

Figure 4 |

Figure 5 |

Figure 6 |

Figure 7 |
Citrus
Spreading decline was first reported from Florida in 1928, but R. similis was not identified as the causal agent until 1953. The decline spreads quickly from tree to tree, with thinning foliage in the upper canopy as an early disease symptom (Figure 7). Leaves and fruits are small, the latter dropping early, as branch ends become bare and eventually die back. Root damage is most severe below 25 to 30 cm (10 to 12 inches) and shallower roots may appear unaffected. In the deep sandy soils of Florida, half of the feeder roots might grow 1 to 6 m (3 to 20 feet) deep and be destroyed, causing trees to wilt quickly as soil moisture decreases. Dark lesions appear at points of entry on feeder roots and expand as nematodes burrow through the root cortex. Roots become stunted, with irregular swellings, due to root tip damage and tissue hyperplasia (abnormal increase in cell number).
Black pepper
The main symptoms of yellows, or slow wilt disease of black pepper, are pale yellow leaves that droop and then fall from the vine. Other symptoms caused by decreased water and nutrient uptake are slow plant growth, flower drop, and vine dieback. Symptoms are more pronounced during dry periods, but if moisture becomes available early in the disease (e.g. tropical monsoon rains) leaves are replaced and vines appear to recover (Figure 8). In 3 to 5 years, however, the disease will reemerge, and so the name ‘slow wilt’. Burrowing nematode attacks both young and old plants, so vines replanted in infested soil normally die within 2 years. Thin, white feeder roots have purplish lesions and are quickly destroyed. Lesions are harder to see on older, brown roots, which are slower to rot.
Morphology of R. similis
The signs (visible presence of the pathogen) of these diseases are the various stages of R. similis observed in soil and plant root samples. All nematode stages are vermiform (wormlike), colorless and less than 1 mm in length. Adult males and females are different in appearance (sexual dimorphism), the males having poorly developed stylets and a knob-like head caused by an elevated, constricted lip region (Figure 9). Both males and females have long, tapered tails with rounded or indented ends (Figure 9). The male has a sharp, curved spicule (male reproductive organ), enclosed in a bursa, or sac (Figure 10). Females are between 550 and 880 µm (0.55 to 0.88 mm) in length and about 24 µm in diameter (Figure 11), with well-developed stylets 16 to 21 µm (average 18 µm) long (Figure 12). Males are smaller than females, 500 to 600 µm in length (Figure 13). Juveniles are often present in both root and soil samples and average between 315 to 400 µm in length with stylets 13 to 14 µm long.

Figure 8 |
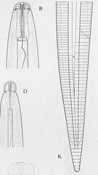
Figure 9 |
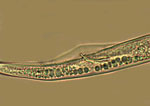
Figure 10 |

Figure 11 |

Figure 12 |

Figure 13 |
Pathogen Biology
Radopholus similis (Nematoda, Tylenchida, Pratylenchidae) was first observed by Nathan A. Cobb in necrotic banana roots from Fiji in 1891. The species is divided into at least two races, a banana race able to parasitize all hosts except citrus and a citrus race that feeds on citrus and other hosts, including banana. In 1984 the citrus race was proposed as a separate species, R. citrophilus. This was rejected when the ability of the two races to mate and produce offspring was established in 1997 and a molecular analysis of their genomes in 2000 determined they were not distinct species. Three other Radopholus species with a limited distribution and host range are: R. musicola on bananas in Australia, R. citri on citrus in Indonesia, and R. bridgei on turmeric, also in Indonesia.
The burrowing nematode is an obligate parasite and needs a living host to survive, though its various stages can move from root to soil and vice versa. It is classified as a migratory endoparasite, completing its life cycle as it tunnels through the root cortex (Figure 14). Females and juvenile stages are infective but males with their weak stylets do not feed. Radopholus similis usually penetrates roots near the tip, but can invade along the entire length of the root. They move between cells of the root cortex, feeding on them until the cells collapse and form necrotic passages. In most hosts, R. similis does not damage the central cylinder (Figures 3, 5), though the citrus race reportedly feeds on the phloem, girdling and destroying the stele. Migration and egg-laying are stimulated by nutritional factors: females need healthy tissue to feed on, but eggs are laid in root tissue that quickly decomposes. Females usually reproduce sexually, but can also reproduce without males. This phenomenon was once considered parthenogenesis, but recent studies suggest it might be hermaphrodism. Females lay 4 to 5 eggs per day (2 per day in citrus) for several weeks as they move through the root cortex.

Figure 14 |

Figure 3 |

Figure 5 |
Disease Cycle and Epidemiology
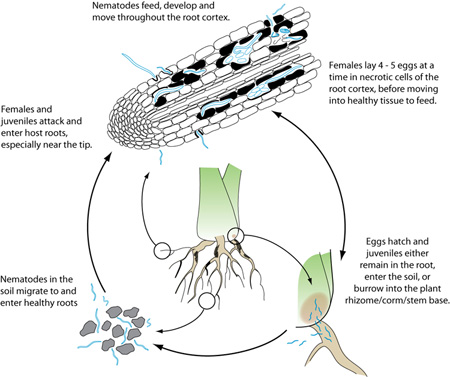
Disease Cycle
The life cycle of R. similis in banana roots, from egg to egg, occurs in 20 to 25 days at 24 to 32°C (75 to 90°F). Eggs are reported to hatch in 8 to 10 days with juvenile stages completed between 10 and 13 days. As with all nematode species, there are four juvenile stages: the first stage (J1) develops within the egg, then molts and emerges as a J2 nematode. In citrus, the life cycle is 18 to 20 days with egg hatch reported between 2 and 3 days.
Since the beginning of the 1800s, the wide distribution of R. similis in tropical and subtropical regions has followed the transfer of infected plant material between countries. Banana is vegetatively propagated and infected suckers and rhizomes continue to be the main method of nematode distribution within and between fields. When roots rot, nematodes either move out into the soil and actively invade nearby healthy roots, or continue feeding into the rhizome (banana), corm (taro), or lower stem (anthurium) of their hosts. Though populations in the soil are usually low, burrowing nematodes can be passively transported in soil water or infested soil clinging to roots, tools, machinery, footwear, et cetera.
Radopholus similis is short-lived in the soil, six months or less. As with all biotrophic organisms, it depends on living host plants for survival (Figure 15). These plants include the current crop, volunteers and weeds. In India, for example, nematode populations in black pepper plantations are sustained by the practice of interplanting with alternative hosts, such as coconut, banana, turmeric and ginger. In the South Pacific, taro, banana and coconut are host to R. similis (Figure 16).

Figure 15 |

Figure 16 |
Root rot initiated by burrowing nematode is often increased by the invasion of other soilborne microorganisms. For instance, the fungi Cylindrocarpon musae and Rhizoctonia can increase damage to banana roots infected by R. similis. It is also known to interact with weakly pathogenic isolates of another fungus, Fusarium solani, increasing root rot and death of black pepper seedlings.
Disease Management
Management of R. similis in the tropics and subtropics is complicated by several factors. First, the nematode’s most important agricultural hosts are perennial crops, making eradication or reduction of nematode populations difficult and expensive. In addition, subsistence agriculture is practiced in most developing countries in these latitudes so fallowing, crop rotation, clean planting material, or nematicidal chemicals may not be practical, affordable, or attainable. Finally, economic thresholds (nematode population at or above which crop loss would cost more than management efforts) for many of these crops have not been established. As a result, crop damage and high nematode populations may occur before the problem is addressed. Nevertheless, there are actions available to exclude, eradicate and avoid R. similis, or to protect crops growing in infested soils.
Exclusion
Efforts between governments to exclude plant pathogens by quarantine are essential, but expensive and often defeated. Radopholus similis is present internationally in most areas where its host plants are grown, though some races are not widespread. For example, rooted citrus from Florida must currently be certified free of burrowing nematode (citrus race) before it can be accepted for import and distribution within the European Union (EU) or other citrus-growing states like California. However, the citrus race is not morphologically or genetically distinct, so all R. similis races are quarantined by the EU. Local contamination of clean fields and greenhouses can be prevented by using nematode-free planting material (Figure 17) and controlling the movement of infested soil into new areas on tools, machinery, humans, or in soil or surface waters. Nurseries must use nematode-free planting material and maintain strict sanitary practices, including disinfesting pots and benches (Figure 18).

Figure 17 |

Figure 18 |
Eradication
Destroying all burrowing nematodes in an area is theoretically possible, but practically difficult. Eradication requires removal of all belowground parts of the host crop, volunteers, alternative hosts, and all weed hosts: R. similis does not form survival structures and will eventually starve in the soil. The “push and treat” method for nematode control in citrus begins with bulldozing and removal of trees, roots and all, followed by nematicide and herbicide applications to reduce or eliminate host plant and nematode population growth. A restriction on the use of nematicides and their degradation by soil microorganisms, however, has reduced the effectiveness of this approach. Host removal is followed by either a clean fallow of 12 months, rotation with non-host crops, or planting a nematode-suppressive cover crop (e.g. Crotalaria). An efficient method of crop destruction in banana plantations is to inject pseudostems with the herbicide glyphosate. This causes roots and rhizomes to die and rot without the need to dig them up. Flooding the field for 8 weeks is effective but expensive and seldom practiced.
Other nematode species such as root-knot (Meloidogyne), lesion (Pratylenchus), citrus (Tylenchulus semipenetrans), or spiral (Helicotylenchus) nematode often occur with R. similis and their populations may increase if R. similis is eliminated.
Avoidance
Damage by R. similis can be avoided by planting disease-free crops in uninfested soil or a clean, soilless potting medium. Injury may be minimized in previously infested fields by planting after the eradication procedures mentioned above (e.g. fallow, crop rotation) have reduced nematode populations in the soil.
Protection
Cultural practices that maintain plant health and vigor may improve yield by allowing plants to rapidly replace roots damaged by R. similis. Incorporating organic material into the soil provides nutrients and improves its cation-exchange and water-holding capacity, which may also increase the number of beneficial microorganisms and nematode antagonists in the root zone. Some cover crops, like sunnhemp (Crotalaria) or marigold (Tagetes), produce chemicals that are toxic to nematodes (allelopathy) and may reduce burrowing nematode populations in the soil.
Soil fumigation with methyl bromide, 1,3 dichloropropene, or chloropicrin can provide good control of burrowing nematodes but these fumigants must be applied pre-plant. Nonfumigant nematicides such as aldicarb and phenamiphos can be used post-plant and usually give systemic plant protection and reasonable control of burrowing nematodes. Chemical control of nematodes in general is decreasing in the U.S., however, due to regulatory restrictions, costs, and environmental concerns. Use of the important pre-plant fumigant methyl bromide, for example, has been restricted to a limited number of crops due to its effect on the ozone layer. Most nonfumigant nematicides are neurotoxic and can also affect humans and useful microorganisms. Some of these concerns may not be regulated in other growing regions of the world where burrowing nematodes exist and nematicide use in these locales can be relatively high. For example, nonfumigant nematicides are routinely applied in commercial banana plantations worldwide, increasing production up to 250%. Nematicides will not kill all parasitic nematodes in the soil and roots, so must be reapplied periodically. With repeated use, however, soil microorganisms become more efficient at degrading nematicides, making them less effective. The high cost of nematicides also limits their use by small growers.
Biological controls of plant-parasitic nematodes target different stages of nematode development using unique modes of action. The fungi Paecilomyces lilacinus and Trichoderma atroviride destroy nematode eggs, while toxic metabolites from non-pathogenic strains of Fusarium oxysporum inhibit or kill juveniles. Mycorrhizal fungi appear to compete with plant-parasitic nematodes for nutrients, such as phosphorus. The bacterium Pseudomonas kills juveniles and adults by producing lethal hydrogen cyanide. Conversely, Pasteuria spp. are bacterial hyperparasites (organisms that parasitize other parasites) that kill nematodes after attaching endospores to their outer cuticle. Despite their potential, the use of biological controls for plant-parasitic nematodes is quite limited at present.
Resistant varieties, when available, are central to any integrated nematode management strategy. Resistance to R. similis is present in some banana hybrids, but their commercial qualities are still not equal to standard cultivated varieties. There are no black pepper varieties resistant to burrowing nematode, so favored cultivars are sometimes grafted onto the nematode-resistant rootstock of Piper colubrinum, a wild relative. Citrus and tea cultivars of high quality and yield are also grafted onto resistant rootstocks. The economic and environmental benefits of developing plant resistance to nematodes cannot be overemphasized and are a compelling area of research.
Significance
Temperate climate nematology had its beginnings in 1743 with discovery of the wheat seed gall nematode, Anguina tritici, by John T. Needham. In contrast, one of the first plant-pathogenic nematodes discovered from the tropics, Radopholus similis, was not described until Nathan A. Cobb found it in bananas from Fiji almost 150 years later. Most tropical and subtropical nematology laboratories were not established nor local problems addressed until the latter half of the 20th Century. This late start accumulating basic information, including the presence of burrowing nematode and the need for quarantine regulations, furthered the worldwide spread of R. similis. The number of management strategies also lags behind those for temperate climate nematodes.
Bananas rank first among fruits produced worldwide and are annually worth about US$2.5 billion. However, only about 10% of the almost 86 million tons produced are traded commercially. The remaining 90% are grown and consumed by subsistence farmers and their families in developing tropical and subtropical regions. Burrowing nematode damage can cause yield losses of 30 to 60% on banana and a reduction in the productive life of the farm. Economic losses due to R. similis are sometimes difficult to assess as it often exists with other plant-pathogenic nematode species such as root-knot (Meloidogyne), lesion (Pratylenchus), or spiral (Helicotylenchus) nematode. Populations of root-knot and lesion nematodes, both of which feed inside plant roots, may increase if R. similis populations decrease.
The citrus race of R. similis has a limited distribution, including Florida, parts of the Caribbean, Cote d’Ivoire and Guyana; R. citri is restricted to Indonesia. Spreading decline in Florida was apparently responsible for yield losses of 40 to 70% in oranges and 50 to 80% in grapefruits in the mid-1900s. During a 10-year period, this accounted for a difference of 1338 versus 62 boxes/ha harvested from healthy versus infested groves, respectively. Exclusion management practices, pull and treat programs, and movement of the Florida citrus industry to new lands within the state have reduced the impact of burrowing nematode in Florida citrus. Economic loss from declining citrus trees in Indonesia has not been determined, but is thought to be substantial.
In black pepper plantations, diseased plants on trellis poles create a forest of yellow leaves and naked vines. Yellows disease was associated with the loss of 22 million pepper vines over a 20-year period in Indonesia and slow wilt was responsible for the death of 10% of vines in parts of India in the early 1900s.
Local, state and international quarantine regulations are a significant economic consequence of burrowing nematode disease. Preshipment certification is often needed for plant export from infested areas to noninfested areas. This includes shipping rooted citrus from Florida to California, for example, or potted anthuriums from Hawaii to California and Japan. Certified nurseries also need a phytosanitary inspection and certificate for each shipment. Costs associated with compliance to quarantine regulations seriously impact growers and consumers on both sides of the law. Preventing the spread of burrowing nematode to new growing areas, however, remains a critical part of its management.
