DISEASE: Cercospora leaf spot (CLS) of table beet
PATHOGEN: Cercospora beticola Sacc.
HOSTS: Primary hosts: table and sugar beet (Beta vulgaris L. subsp.
vulgaris), and Swiss Chard (B. vulgaris L. subsp.
cicla) and spinach (Spinacia oleracea L.). However, the pathogen also infects other plants, such as lambsquarters (Chenopodium album), wild mustard (Brassica kaber), lettuce (Lactuca sativa), safflower (Carthamus tinctorius 'Centennial'), and dock weed (Rumex obtusifolius).

A,
Defoliation of table beet plants caused by Cercospora leaf spot in a small-plot, replicated trial at Freeville, New York in 2019 (nontreated plots in foreground marked by white flags);
B,
Healthy canopy of a table beet field in western New York in 2018; and
C, Table beet roots (cv. Ruby Queen). Images courtesy S. J. Pethybridge.
|
Cercospora leaf spot (CLS), caused by the fungus,
Cercospora beticola, is a widespread disease where table beet, sugar beet, Swiss chard and spinach are grown. The disease causes annual epidemics that spread rapidly and are favored by periods of high temperature (>20°C) and relative humidity (above 90%) or consecutive days of leaf wetness. CLS lesions on the foliage cause reductions in quality and render produce unsuitable for fresh market sale or require foliage removal incurring additional post-harvest handling and cost. The reductions in green leaf area associated with CLS may limit carbohydrate production and affect root sizing and other quality parameters including post-harvest storage. In severe epidemics, defoliation may occur which makes table beet grown in broadacre fields unable to be harvested using top-pulling machinery. Top-pullers remove roots from the ground by grabbing plants from the base of the leaves (Fig. 1). Defoliation from CLS and other factors weakens the leaves resulting in roots that cannot be removed from the soil and significant crop loss. In sugar beet, CLS loss occurs primarily through a reduction in sugar content, root yield, and storage quality.

Fig. 1.
A,
A table beet harvester in western New York that relies upon sufficient healthy foliage to enable top-pulling of roots from the ground; and
B,
Close-up of the 'fingers' of the harvester which uses rotating belts to grab the leaves to remove the roots from the ground. Images courtesy S. J. Pethybridge and J. R. Kikkert.
|

Fig. 2. A,
Cercospora leaf spot epidemics usually begin in table beet fields as small, randomly-distributed foci;
B,
Symptoms on table beet begin as small, gray, circular lesions;
C,
CLS lesions spread rapidly and encompass the entire leaf. Images courtesy S. J. Pethybridge.
|
Symptoms and Signs
Cercospora beticola primarily infects aboveground plant parts, although the pathogen has been reported to colonize sugar beet through the roots. Epidemics typically begin in small, random foci across fields (Fig. 2A). Symptoms first appear as small, gray circular lesions on leaves and petioles (Fig. 2B). Under conducive conditions, necrotic spots enlarge and coalesce to form bigger lesions leading to defoliation (Fig. 2C). CLS symptoms typically first appear in older leaves followed by younger leaves as the disease progresses. In New York table beet fields, CLS lesions typically first appear around mid-July onwards.
Typical necrotic lesions in table beet foliage are gray to brown but have a distinctive gray center and are surrounded by purple (red cultivars,
Figs. 3A and 3B) to tan (yellow and white cultivars,
Figs. 3C and 3D) margins. Colored margins of CLS lesions are absent in sugar beet and spinach.
The pathogen produces signs called pseudostromata within lesions that are visible with a hand lens or a dissecting microscope. Pseudostromata are formed of a thickened mass of mycelia and appear as small, black round structures within the lesion (Fig. 4A). Asexual spores called conidia are produced on conidiophores that emerge through the pseudostromata (Fig 4B). Conidiophores are smooth, erect, sub-cylindrical, uniform in thickness, pale-brown to sub-hyaline (Fig. 4C). Conidia are filiform to acicular, elongated and curved, hyaline (Fig. 4D). Conidia range from 27 to 250 × 2 to 5 μm with 3 to 28 septa. Conidia are usually produced within 10 to 14 days after infection. Sporulation (production of conidia on the lesions) give lesions a gray appearance on the brown necrotic lesions. Conidia can be identified by the presence of a hilum which is the point of attachment to the conidiophores. The presence of pseudostromata within a lesion are diagnostic for CLS and differentiation from other diseases that may also occur on table beet foliage.
Symptoms that may be confused with CLS include bacterial leaf spot caused by
Pseudomonas syringae pv.
aptata and damage due to abiotic factors. Bacterial leaf spot symptoms are often observed early in the season until approximately six true leaves. Lesions are black, generally occur at the leaf margins, are associated with deformation of the leaf, and do not have pseudostromata (Fig. 5). Phoma leaf spot caused by the fungus,
Phoma betae is also commonly found on table beet, generally at higher prevalence and incidence in organic fields. Phoma leaf spot is characterized by concentric rings of pycnidia within the lesions. Pycnidia are specialized asexual fruiting structures containing conidia (asexual spores) within them and can be spherical or oval shaped, while pseudostromata are compact solid structures formed from mass of melanized hyphae on which conidiophores are borne.
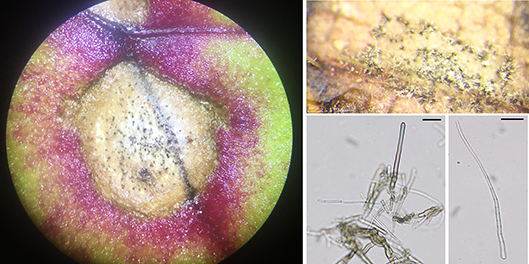
Fig. 4.
Signs of
Cercospora beticola:
A,
Pseudostromata,
B
and C,
Asexual spores (conidia), and the
D, Hilum attaching the conidia to the conidiophores. Images courtesy S. Sharma.
| 
Fig. 5.
A,
Symptoms of bacterial leaf spot caused by
Pseudomonas syringae
pv.
aptata,
and
B,
Phoma leaf spot caused by
Phoma betae.
Images courtesy: S. J. Pethybridge.
|
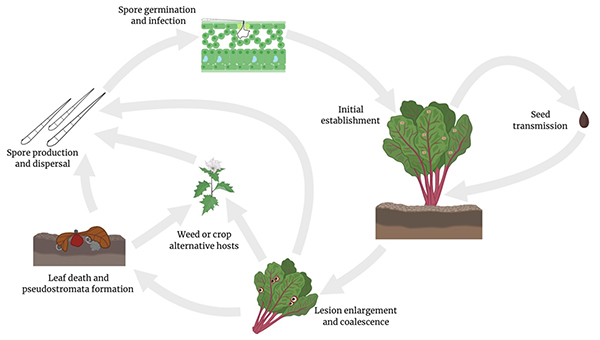
Fig. 6. Lifecycle of
Cercospora beticola, cause of Cercospora leaf spot of table beet. Image courtesy S. Sharma
|
Pathogen Biology
Cercospora beticola is a hemibiotroph and belongs to Class Dothideomycetes, Order Capnodiales, Family Mycosphaerellaceae within the phylum Ascomycota. The fungus propagates through the production of conidia, borne on conidiophores, which are responsible for local or short-range spread. The fungus is generally accepted to be heterothallic with two alternative forms (known as mating-type I and II). Isolates with different mating type loci have been identified from table beet fields of New York and other parts of the United States, and Europe, but the sexual morph has not been observed. Equal frequencies of the two mating-type alleles in table beet fields in New York have suggested the presence of cryptic sex in the lifecycle of
C. beticola. Moreover, characterization of
C. beticola populations using microsatellite markers typically find high genotypic diversity and linkage equilibrium of loci, which suggests sexual recombination within local populations. Knowledge of the potential for long-distance dispersal of the fungus through sexually-produced spores is currently an important research topic to enable the design of durable integrated management strategies to prevent crop losses.
Disease Cycle and Epidemiology
Disease Cycle

Fig. 7. Table beet foliage is returned to the soil surface during harvest to form the crop residue which could harbor Cercospora beticola and serve as inoculum for Cercospora leaf spot epidemics. Image courtesy: S. J. Pethybridge.
|
The primary sources of inoculum for
C. beticola include infested plant debris, alternative hosts, and potentially infested seed. Crop and plant residue that remain in the field following harvest (Fig. 7) may be colonized by the pathogen, which subsequently overwinters (survives between cropping seasons) by forming pseudostromata, which give rise to conidia at the beginning of the growing season to initiate new infections. The pathogen survives in infested plant debris for up to 3 years. The pathogen can also survive on dead plant tissue as a necrotroph regardless of host status. Conidia can be dispersed by wind or rain splash usually resulting in short-range, local movement (within the same field or adjacent fields). Infested crop debris may therefore contribute to the CLS epidemic by splash dispersal of inoculum to the developing crop from the soil surface. Alternative hosts consisting of weeds and other crop species may also be a reservoir for inoculum and form a green bridge for the pathogen to survive between cropping seasons. The disease is polycyclic, meaning that multiple infection cycles can occur within a growing season resulting in rapid disease spread. Direct infection of sugar beet roots has also been reported, and root lesions associated with infection byC. beticola have been described but are considered rare and not reported in table beet. Potential sources of inoculum from outside the field of interest include airborne ascospores from a currently undescribed sexual morph, insects, and infested seed.
Infection begins when conidia reach the surface of leaves and penetrate through stomata in a symptomless biotrophic manner. The fungus then grows intercellularly, colonizing the parenchymatous layer. However, within a few days,
C. beticola becomes necrotrophic and produces phytotoxins that destroy the plant cells and result in disease symptoms. Light is not a prerequisite for infection, but symptoms are associated with a photoactivated phytotoxin called cercosporin. Under controlled environment conditions, a lesion can appear as early as 3 days after infection. Expansion of the lesion requires temperatures between 26 and 32°C and high relative humidity or extended periods of leaf wetness, after which hyphae exit the leaves via stomata and form pseudostromata.
Disease Management
Table beet production in New York is diverse with production systems ranging from small farms, mostly catering to fresh market sales, to broad-acre fields for the processing industry. Either of these production systems may be conventional or organic. Therefore, the in-season control tactics for CLS management are largely dependent on the production system.
Cultural Control
Cultural control forms an important part of the integrated disease management strategy for CLS in conventional and organic production systems. The primary objective of cultural control is to reduce the overwintering inoculum on weeds or alternative hosts that form a green bridge to initiate epidemics in subsequent seasons. Cultural control techniques may take the form of managing crop rotation with non-hosts, control of weeds that may be alternative hosts, residue management, and using certified seed. As
C. beticola survives on crop residue, rotation with non-susceptible hosts (e.g. snap bean, soybean, corn, cabbage) for three years is recommended to decrease the probability of inoculum surviving to initiate a CLS epidemic in the next susceptible crop grown at that location. However, in practice, crop rotation alone is often insufficient to make a difference to in-season disease management.
Weeds may also be major reservoirs of
C. beticola. Several commonly occurring weed species such as dock weed (Rumex obtusifolius), lambsquarters (Chenopodium album), wild mustard (Brassica kaber), and pigweed (Amaranthus retroflexus) are alternative hosts for
C. beticola. Management of weeds within and surrounding crops is therefore encouraged to reduce the probability of pathogen perpetuation while growing non-susceptible crop hosts.
Crop residue management has a strong influence on inoculum perpetuation between susceptible crops. Removal of crop residue (foliage) that remains after harvest from the field is not practical nor economically feasible. As
C. beticola can produce pseudostromata and sporulate on infested crop residue, burying plant debris through plowing or other techniques to enhance residue break-down is recommended. However, tillage of residue to reduce the contribution of soilborne inoculum to CLS epidemics is largely incompatible with many of the tactics adopted by growers to reduce soil erosion and improve soil health.
Manipulating microclimatic conditions within the canopy is also unlikely to be practical for disease management because plant populations and arrangement strongly regulate root shape and size specifications which are important for processing. Infested seed may also be a potential source of
C. beticola in table beet. The use of certified seed and fungicide seed treatments to prevent CLS and other seed or soilborne diseases is highly recommended.
Genetic Resistance
Ruby Queen (a red open-pollinated cultivar) is the most widely grown table beet cultivar in farms in New York. In replicated trials, Ruby Queen had lower incidence and severity of CLS than other red (Detroit, Rhonda, Falcon and Merlin), and yellow (Touchstone Gold and Boldor) cultivars. The identification of table beet cultivars with enhanced CLS resistance is a priority and a primary goal of public and private breeding programs. The release and adoption of new cultivars is also complicated by strict industry standards for processing, such as root color, size and shape.
Fungicides
In-season control of CLS relies primarily on fungicides which are generally applied following the first appearance of symptoms. Early diagnosis and preventive measures significantly restrict disease spread and reduce the number of infection cycles per season. Based on modes of action, fungicides are classified into groups according to the Fungicide Resistance Action Committee (FRAC). Products and their FRAC groups currently approved for CLS management on table beet in New York are listed in
Table 1. These include single-site mode of action fungicides belonging to FRAC group 3 (demethylation inhibitors), 7 (succinate dehydrogenase inhibitors), 9 (anilinopyrimidines), 11 (quinone outside inhibitors), and 12 (signal transduction inhibitors).
Table 1. Conventional site-specific mode of action fungicides currently registered for Cercospora leaf spot control in table beet in New York.
|
Product |
Active ingredient(s) |
FRAC group(s) |
| Quadris | Azoxystrobin | 11 |
Reason
| Fenamidone | 11 |
Cabrio
| Pyraclostrobin | 11 |
| Flint Extra | Trifloxystrobin | 11 |
Luna Tranquility
| Fluopyram + pyrimethanil | 7 + 9
|
| Luna Sensation | Fluopyram + trifloxystrobin | 7 + 11 |
| Merivon | Fluxapyroxad + pyraclostrobin | 7 + 11 |
| Tilt | Propiconazole | 3
|
| Miravis Prime | Pydiflumetofen + fludioxonil | 7 + 12
|
High allelic and genetic diversity typical of
C. beticola populations enables the pathogen population to rapidly change in response to selection pressures such as single site mode of action fungicides.
C. beticola is therefore considered a high-risk pathogen for fungicide resistance. Resistance within
C. beticola populations to azoxystrobin (Quadris) and other strobilurin fungicides within FRAC group 11 is common. FRAC group 11 resistance was responsible for in-field failures of CLS control in New York in 2012. Strobilurin fungicides had been central for the management of CLS and pocket rot, caused by the fungus
Rhizoctonia solani, for many years. Failure of strobilurins to control CLS in the field, and characterization of resistance of subsequently isolated
C. beticola to these active ingredients
in-vitro has made them less a desirable option for disease management. Resistance to propiconazole (Tilt) and other demethylation inhibitors (FRAC group 3) have also been reported in
C. beticola populations on table and sugar beet, suggesting their long-term use is unsustainable. Resistance to benzimidazoles and tin-based products have also been reported within
C. beticola populations in the United States. These products are not registered for use in table beet.
Multisite fungicides such as copper-based products are of moderate efficacy for CLS control and need to be applied with care to avoid phytotoxicity as CLS sprays often coincide with high temperature and light conditions. Relying on multisite protectants generally reduces the selection pressure on a pathogen population but requires more intensive use patterns. Copper-based fungicides may also have more environmental and off-target impacts than single-site fungicides. Organic Materials Review Institute (OMRI)-approved products that provide CLS control in table beet and offer FRAC group rotation include microbial-based products such as
Bacillus amyloliquefaciens (e.g. Double Nickel, FRAC group 44) and
B. mycoides (e.g. LifeGard, FRAC group P6). Rotation of fungicides belonging to a different FRAC group is recommended wherever possible for effective control and to abide by best management practices for fungicide resistance.
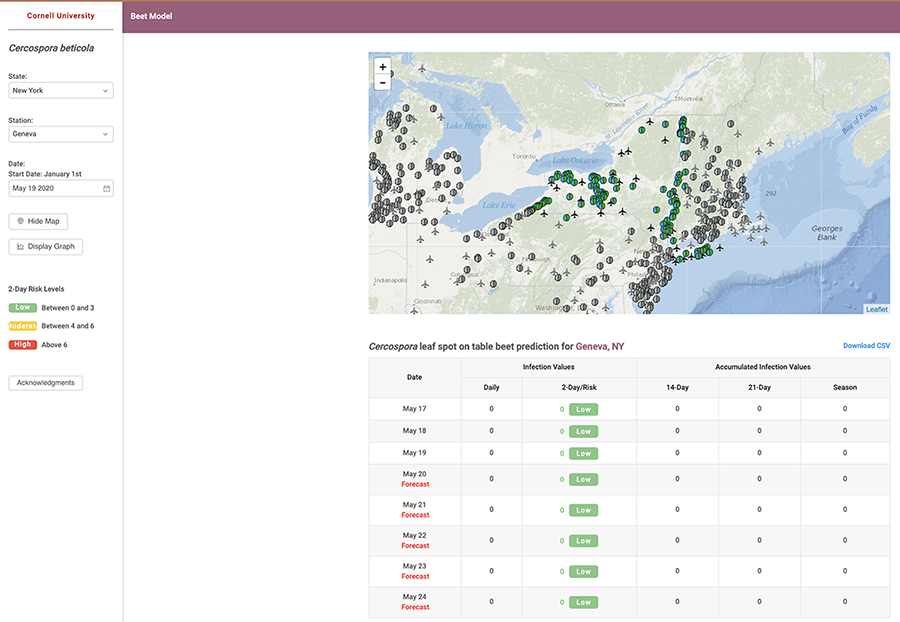
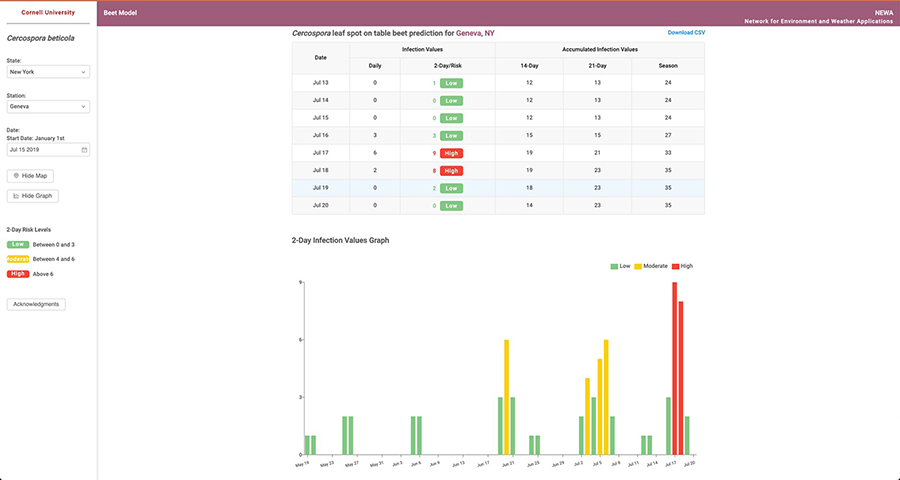
Fig. 8. Cercospora leaf spot forecasting system dashboard images depicting A, Daily infection values and accumulated infection values during May 2020, and B, A graphical representation of the values with low, medium, and high in July 2020.
|
Timing of fungicide application and ensuring adequate coverage is crucial for CLS management. Judicious application of fungicides is essential to minimize the risk of resistance development to single-site mode of action fungicides. This can be achieved by improved scheduling and the integration of epidemiological information to assess disease risk. Based on a CLS decision support system for sugar beet in the mid-western US, a weather-based disease forecaster has been adopted for CLS management in table beet in New York (
Fig. 8). The CLS decision support system can be accessed through the Network for Environment and Weather Applications (NEWA; New York State Integrated Pest Management Program, Cornell University, Geneva, NY and the Northeast Regional Climate Center, Ithaca, NY) online at
https://alexsinfarosa.github.io/beet-model/. The model is based on temperature and relative humidity monitored 1.5 m above the soil. Local weather stations take daily measurements and are linked to NEWA. The temperature and relative humidity measurements are used to calculate daily infection values (DIVs), which in turn, are accumulated to determine the daily infection risk (DIR). DIR values are categorized into different risk levels that can be used to guide decisions for the application of fungicides (
Table 2). They are used to inform crop scouting and decisions to apply fungicides. Scouting usually begins at canopy closure (~90% of plants from adjoining rows touching) and continues on a weekly basis thereafter. The combination of temperature and rainfall in the previous 48 hours is integrated based on the cardinal epidemiological data available for
C. beticola on sugar beet into daily infection values (DIVs), which range from 0 to 7. If the sum of DIVs from two consecutive days is less than 6, equal to 6, or more than 6, the potential for infection is considered unfavorable, marginal, or favorable for infection, respectively.
Table 2. Daily infection risk value ranges for Cercospora leaf spot of table beet based on the decision support system.
|
Daily infection risk values |
Risk category |
Color designation |
| 0 to 3 | Slight
| Green |
4 to 6
| Moderate
| Yellow |
| 7 to 14 | High | Red
|
Significance
Cercospora beticola Sacc. is the most destructive foliar pathogen of selected Chenopodiaceae crops, including table beet and sugar beet. The disease was first reported by Saccardo (1876) followed by a detailed description of the disease and pathogen by L. H. Pammel in 1888. The disease was noted as a serious concern in beet-growing regions worldwide in the late 1800s. At that time, removal and burning of the diseased plant material was the recommended practice with the application of Bordeaux mixture and copper-based products.
The genus
Cercospora comprises one of the largest groups in the Dothideomycetes and is linked to the teleomorph genus
Mycosphaerella.
Cercospora beticola has been one of the most well-studied organisms in the genus and serves as a model organism to dissect stomatal tropism, virulence and cercosporin production within
Cercospora. This has been facilitated by the availability of the high-quality genome assemblies of several isolates and availability of tools for genetic manipulation.
C. beticola is amenable to
Agrobacterium- and PEG mediated transformation. Recent work on the cercosporin gene cluster in
C. beticola has shed new light on the expansion of the cluster and relevance in other genera such as
Colletotrichum. Additional work to understand effector biology in
C. beticola could disentangle virulence mechanisms and help augment breeding efforts towards durable disease resistance in table and sugar beet.
The broad host range of
C. beticola also contributes to its economic importance and complicates management. In table beet, loss of foliage due to CLS impedes the ability to harvest using top-pulling machinery. This causes substantial loss to the table beet industry in New York, which is currently the second largest table beet producing state in the United States and accounts for $1.8 million and $8.47 million for industry and fresh market, respectively. In sugar beet, the estimated annual loss due to reduced yield and fungicide application by
C. beticola is $45 million. In addition to yield reductions, CLS also causes significant decreases in extractable sugar content due to loss of photosynthetic capacity and carbohydrate production.
de Jonge, R., Eberte, M. K., Huitt-Roehlh, C. R., Palh, P., Suttle, J. C., Spanner, R. E., Neubauer, J. D., Jurick, W. M., Stott K. A., Secor, G. A., Thomma, B. P. H. J., de Peer, Y. V., Townsend, C. A., and Bolton, M. D. 2018. Gene cluster conservation provides insight into cercosporin biosynthesis and extends production to the genus
Colletotrichum. Proc. Natl. Acad. Sci. 115:E5459-E5466.
Fungicide Resistance Action Committee. 2020.
FRAC Code List. Accessed July 2020.
Groenewald, J. Z., Nakashima, C., Nishikawa, J., Shin, H.-D., Park, J.-H., Jama, A.N., Groenewald, M., Braun, U., and Crous, P. W. 2012. Species concepts in
Cercospora: spotting the weeds among the roses. Stud. Mycol. 75:115–170.
Knight, N., Vaghefi, N., Kikkert, J., and Pethybridge, S. J. 2019. Alternative hosts of
Cercospora beticola in field surveys and inoculation trials. Plant Dis. 103:1983-1990.
Pethybridge, S. J., Vaghefi, N., and Kikkert, J. 2017 Horticultural Characteristics and Susceptibility of Table Beet Cultivars to Cercospora Leaf Spot in New York. HortTechnol. 27:530-538.
Pethybridge, S. J., Kikkert, J., Hanson, L. E., and Nelson, S. C. 2018. Challenges and prospects for building resilient disease management strategies and tactics for the New York table beet industry. Agronomy 8:112; doi:10.3390/agronomy8070112.
Pethybridge, S. J., Sharma, S., Hansen, Z., Kikkert, J. R., Olmstead, D. L., and Hanson, L. E. 2020. Optimizing Cercospora leaf spot control in table beet using action thresholds and disease forecasting. Plant Dis. 104:1831-1840.
Rangel, L., Spanner, R. E., Ebert, M. K., Pethybridge, S. J., Stukenbrock, E. H., de Jonge, R., Secor, G. A., and Bolton, M. D. 2020.
Cercospora beticola: the intoxicating lifestyle of the leaf spot pathogen of sugar beet. Mol. Plant Pathol. 21:1020-1041.
Vaghefi, N., Kikkert, J. R., Hay, F. S., Carver, G. D., Koenick, L. R., Bolton, M. D., Hans on, L. E, Secor, G. A., and Pethybridge, S. J. 2018. Cryptic diversity, pathogenicity, and evolutionary species boundaries in
Cercospora populations associated with Cercospora leaf spot of
Betavulgaris. Fungal Biol. 122:264-282.
