: Downy Mildew of CucurbitsPseudoperonospora cubensis (Berkeley & Curtis) Rostovtsev Members of Cucurbitaceae, the gourd family, including cucumber (Cucumis sativus), squash (Cucurbita spp.), melon (Cucumis melo) and watermelon (Citrullus lanatus).

Symptoms and Signs
Downy mildew affects plants of all ages. Although the disease only infects foliage, a reduction in photosynthetic activity early in plant development results in stunted plants and yield reduction, especially in cucumber. Premature defoliation may also result in fruit sunscald due to overexposure to direct sunlight. Symptoms of downy mildew infection exhibit themselves differently on the various cucurbit crops.
Cucumber, squash and pumpkin.
Symptoms on cucumber and squash are angular lesions that are limited by the leaf veins. During periods of leaf wetness from dew, irrigation or rainfall, incipient lesions can become conspicuously water-soaked (Figure 1). This is the earliest symptom produced by the disease, but will disappear as moisture dissipates. Early lesions are light green in appearance (Figures 2 and 3) and become chlorotic and finally necrotic as host plant cells die (Figure 4). Severe infection results in leaves that are completely dead and curled up. This symptom has been described as “wildfire” as the leaves appear to be burned (Figure 5).

Figure 1 |

Figure 2 |

Figure 3 |

Figure 4 |

Figure 5 |
Watermelon and cantaloupe.
Symptoms on watermelon and cantaloupe are typically irregular shaped lesions on the foliage that turn brown rapidly (Figures 6 and 7). Infected leaves may experience an upward leaf curl. Symptoms on watermelon and cantaloupe are not as distinctive as on cucumber and squash and are more easily mistaken for other diseases such as anthracnose (causal agent:
Colletotrichum orbiculare), target spot (causal agent:
Corynespora cassiicola), Alternaria leaf spot (causal agent:
Alternaria alternata f. sp.
cucurbitae), or gummy stem blight (causal agent:
Didymella bryoniae).
Signs.
The key to identifying downy mildew is observing the signs (sporangia and sporangiophores) of the pathogen. Sporangia and sporangiophores are most noticeable during humid conditions (e.g., early morning hours before natural humidity dissipates or immediately following rainfall) on the underside of the leaf. In very severe infections, sporulation can occur also on the upper leaf surface, although this is uncommon.
Upon inspection, the underside of an infected leaf reveals downy, fuzzy growth, arising within the leaf veins (Figure 8). The hue of the sporulation ranges from colorless to gray-brown to deep purple. The color depends on density and age of the sporangia that darken with age (Figure 9). In the very early stages of disease, sporulation is not apparent to the naked eye, and a microscope is needed. However, an experienced field pathologist can detect low levels of sporulation using a 20X handlens.

Figure 8 |

Figure 9 |
Pathogen Biology
Pseudoperonospora cubensis is a fungal-like organism that belongs in the Kingdom Straminipila and phylum Oomycota.
Pseudoperonosporacubensis is a member of Peronosporaceae (the downy mildew family) in the order Peronosporales within the class Oomycetes. Like other downy mildew organisms,
P. cubensis, is a biotroph or obligate parasite, meaning that the organism requires living host tissue to grow and reproduce. The organism cannot be propagated on artificial media.
P. cubensis overwinters on infected cucurbits, either wild or propagated, in areas that do not experience a hard frost, such as southern Florida in the eastern United States.
Sporangia and sporangiophores.
Pseudoperonospora cubensis forms large (20-40 x 14-25 mm in diameter), lemon-shaped sporangia with a conspicuous papilla. Sporangia appear smooth under the compound microscope (Figure 10), but with the scanning electron microscope the finely decorated surface of the sporangium is evident (Figure 11). The sporangia are borne singly on the pointed tips of sporangiophores that branch at acute angles (Figures 12 and 13). The sporangiophore ranges from 180-600 mm in height, 20 mm in diameter and 5-7 mm in width.
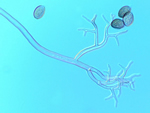
Figure 10 |
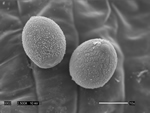
Figure 11 |
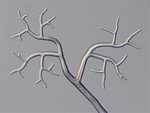
Figure 12 |
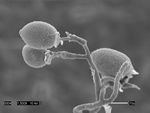
Figure 13 |
Zoospores.
Moisture prompts the sporangia to release 5 to 15 asexual, ovoid zoospores that measure 10-13 mm in diameter. The zoospores are biflagellate, with one posterior whiplash and one anterior tinsel flagellum. The purpose of the flagella is to assist the zoospores as they swim through free moisture on the leaf surface to a stomate. Once a stomate is located, the zoospore will encyst (produce a cell wall) and then form a germ tube (50-95 mm), which will penetrate the stomate and infect the host plant cell.
Intercellular mycelium.
Once a host plant is infected, mycelium grows within and between host cells and serves as the body of the pathogen. The intercellular mycelium is hyaline (colorless, transparent) and coenocytic (aseptate). The mycelium develops in the mesophyll, but also penetrates the palisade tissue. The hyphal diameter is 5.4-7.2 mm. Haustoria are formed within the host cells and allow for the absorption of nutrients. Haustoria are varied in shape and appear stunted, inflated or as branched clusters of hyphae.
Oospores.
The role of oospores in the life cycle
of P. cubensis is unknown. Sexual reproduction and formation of oospores in
P. cubensis are rare. Oospores have only been reported from Russia, China, Japan, India and Italy and their germination was not observed. Oospores could function as survival structures when living hosts are not present, but this has not been demonstrated.
Host
Range. Forty species in 20 genera within the Cucurbitaceae are known to be hosts to
P. cubensis. Downy mildew on
Cucumis spp., including cucumber and true melons, alone has been reported in 70 countries. While it has the widest distribution on all continents on the genus
Cucumis, downy mildew is common on
Cucurbita crops, including squashes and pumpkins, in Australia, North America and the Pacific region and occurs less commonly in Asia and Africa. Downy mildew is virtually absent from
Cucurbita in Europe. Downy mildew is widely distributed on
Citrullus spp., including watermelon, in the Americas but scattered elsewhere and absent in Europe and the Middle East, even though the climate is similar to areas where the disease is a severe problem. The disease is found on
Luffa only in Southeast Asia.
Host specialization within Cucurbitaceae is known to exist. Thomas et al. conducted a study in 1987 that summarized five distinct pathotypes (Table 1). Recent studies suggest that more pathotypes may exist and that host specialization is more varied and dynamic (Colucci, 2008 – see
Selected References).
Table 1. Pathotype designations based on
Pseudoperonospora cubensis and host
compatibility (Thomas et al., 1987).
Table 1.
Host | Pathotype |
1 | 2 | 3 | 4 | 5 |
Cucumis sativus | + | + | + | + | + |
C. melo var.
reticulatus | + | + | + | + | + |
C. melo var.
conomon | ─ | + | + | + | + |
C. melo var.
acidulous | ─ | ─ | + | + | + |
Citrullus lanatus | ─ | ─ | ─ | + | + |
Cucurbita spp. | ─ | ─ | ─ | ─ | + |
+ Highly compatible host interaction, -- incompatible or very slightly compatible host-pathogen interaction.
Nomenclature.
Berkeley and Curtis first documented downy mildew of cucurbits in 1868 in Cuba. The duo named the pathogen
Peronospora cubensis. In 1902 Berlese subdivided the genus
Plasmopara Schroeter, placing
Peronosporacubensis Berk. & Curt. in the subgenus
Peronoplasmopara. The subgenus was based on the branching of sporangiophores, similar to that of
Peronospora (acutely branching dichotomously with pointed tips), and the presence of sporangia that germinate by releasing zoospores, similar to the reproductive unit of
Plasmopara. In 1903 Rostovtsev conducted a critical study of the downy mildew pathogen of cucumber in Russia. Like Berlese, Rostovtsev noted the similarities between the cucurbit downy mildew pathogen and
Peronospora and
Plasmopara. In his report, Rostovtsev proposed the new genus
Pseudoperonospora without mention of Berlese’s 1902 report. Because of Rostovsev’s complete description and intricate drawings, the genus
Pseudoperonospora is the accepted and proper name.
Life Cycle and Epidemiology
Pseudoperonospora cubensis causes a polycyclic disease. Sporangia are the source of primary inoculum. Sporangia are transported from infected plants via wind currents and travel to local or distant places. Optimal temperature for sporulation is 15˚C with 6 to 12 hours of available moisture. Symptomatic plants with yellow lesions have the greatest sporulating capacity. The sporulating capacity of purely necrotic lesions is low and that of yellow-necrotic lesions is intermediate. Sporangia and sporangiophores are greatly affected by changes in temperature and humidity. Warming and drying of the atmosphere, typical of early morning hours, causes twisting of the sporangiophores, which may be of importance for the detachment of sporangia (Figure 14).
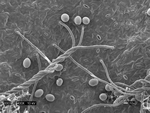
Figure 14 |
Once sporangia land on a susceptible host, free moisture is required for each sporangium to release 5-15 zoospores. Free moisture is also important for zoospore movement, germ tube development and penetration of host tissue by the germ tube. However, excess moisture may reduce the duration of sporangia viability. Zoospores can be released between temperatures of 5 and 28°C. The temperature optimum for zoospore release depends on the duration of the leaf wetness period. If the host leaf remains wet for only 1 hour, the minimum temperature for zoospore release is 10°C, and the greatest amount of spore release occurs at 20°C. If the host leaf remains wet for 2 hours, the minimum temperature is 5°C, and the greatest amount of zoospore release occurs at 15°C.
The optimum temperature for zoospores to form cysts is 25˚C. High temperatures induce immediate cyst formation. Zoospores encyst on a stomatal opening and then form a germ tube that will enter the host via the stomate. Once the host tissue is infected, intercellular hyphae form haustoria within plant cells which provide nutrients for survival and asexual reproduction.
New sporangiophores, differentiated from the mycelium, emerge singly or in groups from the epidermis, usually via the stomata. The new sporangia are produced 4-12 days after initial infection, depending on temperature and day length. Because of the higher frequency of hyphae infecting the spongy parenchyma, the vast majority of sporangiophores and sporangia are produced on the lower leaf surface. High humidity is required for the emergence of sporangiophores.
Symptoms appear 3-12 days after infection, depending on temperature, presence of free moisture and inoculum dose. High temperatures (>35ºC) are not favorable for disease development. However if cooler nighttime temperatures occur, disease development may progress.
Disease Management
Management requires a multi-faceted approach including cultural practices to decrease moisture in the plant canopy, avoidance by changing the planting date, using disease resistant or tolerant varieties and applying effective fungicides. A forecasting system was developed at North Carolina State University to help monitor downy mildew outbreaks in crop production areas and track the movement of sporangia in North America.
Cultural practices.
Downy mildew severity can be decreased by taking actions that encourage airflow and reduce leaf wetness. However, such actions are often insufficient during prolonged, favorable environmental conditions and in the presence of high inoculum levels. Growing cucurbits in environments where humidity levels can be manipulated can help to manage downy mildew. For example, trellising cucurbits, increasing plant or row spacing or growing in passive or traditional greenhouses can help reduce relative humidity and leaf wetness.
In the eastern United States the presence of downy mildew is an annual event in fall cucurbit production. Depending on the latitude of the production area, if a grower has the option to plant earlier in the spring, disease may be largely avoided. For example, in North Carolina pickling cucumbers can often be planted in late-April and harvested before downy mildew has arrived. Conversely, in some mid-Atlantic states, pickling cucumbers are not planted after July because disease pressure is high and the cost of controlling the disease makes profitability much less likely.
Host Resistance.
Most popularly used cultivars of cucumber and cantaloupe, and to a lesser extent squash and pumpkin, have some level of downy mildew resistance bred into them (Figure 15). Even though cultivars with downy mildew resistance may become diseased, disease onset may be delayed, disease may be less severe or the pathogen may produce fewer sporangia than on cultivars without resistance. However, since a new, more virulent strain of
P. cubensis arrived in the eastern United States in 2004, cucumber production cannot rely solely on downy mildew resistant cultivars for control.

Figure 15 |
Chemical Practices.
Chemical control can be very effective at managing downy mildew on cucurbits. However, when chemical control is used in combination with cultural practices, host resistance, and disease forecasting growers can reduce pesticide use and save money. Efficacious fungicides include fluopicolide, famoxadone + cymoxanil, cyazofamid, zoxamide and propamocarb hydrochloride. The results of fungicide trials can be found in Plant Disease Management Reports, a resource published by the Plant Management Network found on-line at
http://www.plantmanagementnetwork.org/pub/trial/pdmr/. Results are also reflected in the Cooperative Extension chemical control recommendations that are issued annually in many U.S. states.
Pseudoperonospora cubensis is known to develop resistance to fungicides very rapidly. Reduced efficacy of mefenoxam, metalaxyl and the strobilurin fungicides has been reported. As a result, the fungicides listed previously should be applied under strict resistance management strategies that include tank-mixing with protectant fungicides, such as chlorothalonil or mancozeb, and rotating with fungicides of different modes of action.
Disease Forecasting.
Early detection is key to managing cucurbit downy mildew. If chemical sprays are not initiated in a timely manner, downy mildew is difficult to manage. In 1998, Holmes and Main of North Carolina State University began a forecasting system to track the outbreaks of the diseases and to provide risk assessments of future outbreaks based on long-distance movement of the pathogen and large-scale weather systems. The goal of the forecasting system is to assist growers in making timely fungicide applications for maximum benefit.
The cucurbit downy mildew forecasting system differs from other disease forecasting systems that assume the host and inoculum are present and efforts are based solely on the environmental factors that favor growth and development of the pathogen (Figures 16 and 17). The cucurbit downy mildew forecasting is currently part of the ipmPIPE (Integrated Pest Management Pest Information Platform for Extension and Education) Project and can be found at
http://cdm.ipmpipe.org.
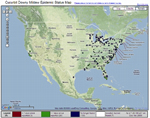
Figure 16 |
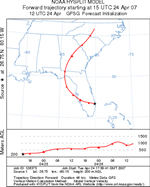
Figure 17 |
Significance
Berkeley and Curtis first reported downy mildew in 1868 from Cuba. Downy mildew of cucurbits can be found in temperate areas, such as the Americas, Europe, Japan, Australia and South Africa, tropical regions internationally and some semi-arid regions, such as the southern United States and the Middle East.
In 1991, 21% of expenses for chemical disease control were allocated to the downy mildew and late blight pathogens, 30% of this was devoted to vegetable crops. In 1996 the global fungicide market was estimated at approximately 6.25 billion USD, of which 16.7% were chemicals to control downy mildews. The largest percentage of downy mildew control was for grape downy mildew caused by
Plasmopara viticola (54%) followed by the cucurbit downy mildew caused by
Pseudoperonospora cubensis (10%). These values fluctuate based on weather conditions, acreage and market conditions.
Downy mildew is an annual late-season problem for squash and pumpkin growers in the eastern United States. Successful breeding of resistant cucumber cultivars in the mid-20th century provided adequate control of downy mildew without the use of fungicides. The presence of a more virulent strain of the pathogen on cucumber appeared in the eastern United States in 2004 and has resulted in major economic losses. In 2004 the eastern United States experienced a major downy mildew epidemic on cucumber that resulted in an economic loss of $16 million USD. The disease is a major concern for cucumber growers throughout the eastern half of the United States resulting in substantial economic losses while also affecting other cucurbit crops.
Selected References:
Cohen, Y. 1981. Downy mildew of cucurbits. In: D.M. Spencer (ed), The Downy Mildews, Academic Press, NY. 636 pages.
Colucci, S. 2008. Host Range, Fungicide Resistance and Management of
Pseudoperonospora cubensis, Causal Agent of Cucurbit Downy Mildew. Master’s thesis. North Carolina State University.
Crute, I.R. 1981. The host specificity of Peronosporaceous fungi and the genetics of the relationship between host and parasite. In: D.M. Spencer (ed.), The Downy Mildews, Academic Press, NY. 636 pages.
Gisi, U. 2002. Chemical Control of Downy Mildews. In: Advances in Downy Mildew Research. P.T.N. Spencer-Phillips, U. Gisi and A. Lebeda (eds.). Kluwer Academic Publishers. Dordrecht. 119-159.
Holmes, G., T. Wehner, and A. Thornton. 2006. An old enemy re-emerges. American Vegetable Grower. February, 2006. 14-15.
Holmes G.J., C.E. Main, Z.T. Keever III. 2004. Cucurbit downy mildew: A unique pathosystem for disease forecasting. Pages 69-80 in: P.T.N. Spencer‑Phillips and M. Jeger (eds), Advances in Downy Mildew Research, vol. 2. Kluwer Academic Publishers, Dordrecht, The Netherlands.
Lebeda, A., and M.P. Widrlechner. 2003. A set of Cucurbitaceae taxa for differentiation of
Pseudoperonospora cubensis pathotypes. Journal of Plant Disease and Protection 110:337-349.
Palti, J. 1974. The significance of pronounced divergences in the distribution of
Pseudoperonospora cubensis on its crop hosts.
Phytoparasitica 2:109-115.
Palti, J. 1975.
Pseudoperonospora cubensis. Descriptions of Pathogenic Fungi and
Bacteria, no. 457. Commonwealth Mycological Institute, Kew, England.
Palti, J., and Y. Cohen. 1980. Downy mildew of cucurbits (Pseudoperonospora cubensis): The fungus and its hosts, distribution, epidemiology and control. Phytoparasitica 8:109-147.
Palti, J., and R. Kenneth. 1980. Distribution of downy mildew fungi over the orders, families and genera and higher plants. In: D.M. Spencer (ed.), The Downy Mildews, Academic Press, NY. 636 pages.
Thomas, C.E., and E.L. Jourdain. 1992. Host effect on selection of virulence factors affecting sporulation by
Pseudoperonospora cubensis. Plant Disease:76:905-907.
Thomas, C.E., T. Inaba, and Y. Cohen. 1987. Physiological specialization in
Pseudoperonospora cubensis. Phytopathology 77:1621-1624.
Waterhouse, G.M. 1973.
Peronosporales. In: G.C. Ainsworth, F.K. Sparrow, F.K. and A.S. Sussman (eds.), The Fungi, an Advanced Treatise. Vol 4B.. Academic Press. New York.
Waterhouse, G.M., and M.P. Brothers. 1981. The taxonomy of
Pseudoperonospora. Mycological Papers No. 148:1-28.
Zitter, T.A., D.L. Hopkins, and C.E. Thomas. 1996. Compendium of Cucurbit Diseases. American Phytopathological Society Press, St. Paul, MN.
