Updated 2017.
Downy mildew
Plasmopara viticola
All cultivars of grapes in the species
Vitis vinifera and many
Vitis intraspecific hybrid cultivars. Susceptibility within the North American species
Vitis labrusca, varies from highly susceptible to resistant.

A typical method of growing grapevines on a trellis system.
(Courtesy G. Ash)
Symptoms and Signs
Although all green parts of the grapevine are susceptible, the first symptoms of downy mildew of grapes, caused by
Plasmopara viticola, are usually seen on the leaves as soon as 5 to 7 days after infection. Foliar symptoms appear as yellow circular spots with an oily appearance (oilspots) (Figure 2). Young oilspots on young leaves are surrounded by a brownish-yellow halo. This halo fades as the oilspot matures. The spots are yellow in white grape varieties and red in some red grape varieties (e.g., Ruby Red). Under favorable weather conditions, large numbers of oilspots may develop and coalesce to cover most of the leaf surface (Figure 3). After suitably warm, humid nights, a white downy fungal growth (sporangia) will appear on the underside of the leaves and other infected plant parts (Figure 4). The disease gets its name "downy mildew" from the presence of this downy growth.

Figure 2 |

Figure 3 |

Figure 4 |
In late summer and early fall, the diseased leaves take on a tapestry-like appearance when the growth of the pathogen is restricted by the veinlets (Figure 5). Confirmation of active downy mildew is made by the "bag test." To do this test, seal suspect diseased leaves and/or fruit bunches in a moistened (not wet) plastic bag and incubate in a warm (13-28ºC/ 55-82ºF), dark place overnight. Look for fresh, white downy sporulation beneath suspect oilspots or on shoots or fruit bunches (Figure 4). Note that mature berries, although they may be symptomatic and harbor the pathogen, may not support sporulation even when provided with ideal conditions. Infected parts of young fruit bunches turn brown, wither, and die rapidly. If infections occur on the young bunch stalk, the entire inflorescence may die (Figure 6). Developing young berries will either die or, if between 3 and 5 mm in diameter, become discolored (Figure 7). Berries become resistant to infection within 2-3 week after bloom, although all parts of the rachis may remain susceptible 2 months after bloom.

Figure 5 |

Figure 6 |

Figure 7 |
Pathogen Biology
The pathogen,
Plasmopara viticola, produces asexual, biflagellate zoospores and sexual oospores. Its mycelium is aseptate. It is an oomycete in the order Peronosporales. Other important downy mildew pathogens that belong to this group include species within the genera
Bremia,
Peronospora and
Sclerospora. Most taxonomists no longer include oomycetes within phylogenetic groupings containing ascomycetes or basidiomycetes, and have placed them instead in the kingdom Chromista. However, phylogenetic considerations aside, oomycetes share many biological, ecological, and epidemiological characteristics with fungal plant pathogens.
Plasmopara viticola is an obligate parasite, and it absorbs its nutrients from the living host tissue via globose haustoria. The hyphae are largely internal in the host. Sexual reproduction occurs through the fusion of antheridia and oogonia within the host tissue.
Plasmopara viticola has only recently been shown to be heterothallic. The resulting sexual spore is an oospore, which is the survival and resting stage of the pathogen.
Oospores represent the primary inoculum, and may overwinter in leaf litter or may be released into the soil as leaves decay or are buried by detritivores. They generally begin to germinate in significant numbers shortly after bud break of grape, and populations of oospores may continue to germinate for the entire growing season in some growing regions. Oospores form a single germ tube terminating in a sporangium. Zoospores form within the sporangia and are then released. Zoospores germinate and penetrate the plant only through functioning stomata, i.e., only on green host tissue. Sporangia and zoospores are easily desiccated. They die within 2 to 3 hr of exposure to low humidity and sunlight, so most infection occurs soon after their release. However, they may survive on leaf surfaces for more than 24 hrs under cool humid conditions.
Sporangia (Figure 8) also serve as a means of secondary spread of the pathogen. Treelike sporangiophores, bearing white, lemon-shaped sporangia, are produced from a mycelial mat within the host tissue and emerge through stomata. Sporangia are disseminated by wind and rain splash. Zoospores released from the sporangia swim in free water (www.youtube.com/watch?v=BKs8WM01iIk) on the grapevine surface, and encyst near a stoma. Zoospores then germinate and penetrate through a stoma by the means of a germ tube. A time lapse video showing sporulation, releasing of zoospores and germinated zoospore can be found at
www.youtube.com/watch?v=qnE91MbZrsg. Oospores are produced in infected host material towards the end of the season. The resulting oospores are thick-walled and serve not only as survival spores during the intercrop period, but are also a source of genetic variation.
Figure 8 |
Disease Cycle and Epidemiology
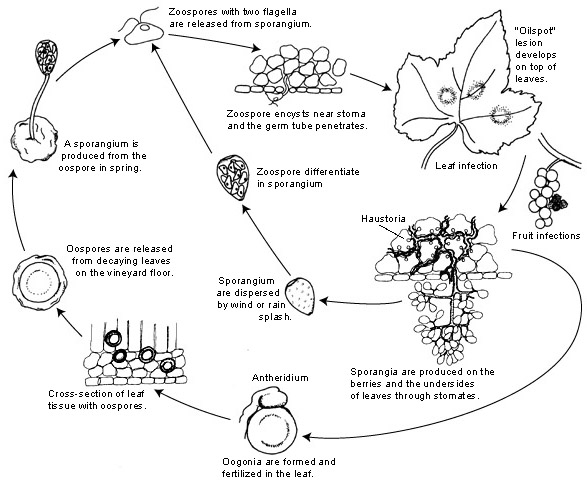 Disease Cycle
Disease Cycle The pathogen survives the winter period as oospores embedded in dead leaves and other host tissue on the vineyard floor. Oospores may be released from the decaying plant material onto the soil surface.
Oospores typically produce sporangia. These sporangia, in turn, produce zoospores. Sporangia and zoospores are splashed by rain or carried by wind to the lower leaves and tissues of the grapevines.
The conditions necessary for oospore germination are wet soils with temperatures above 10ºC (50ºF). An additional rule of thumb in some regions includes rainfall of at least 10 mm (0.04 in.) in a 24-hr period to satisfy the requirement of soil wetness. Once zoospores form and are dispersed to host tissue, infection will occur given a minimum of 45 degree-hours of leaf wetness [“degree hours” is the summation of hourly temperatures (C) until the specified value is achieved]. Zoospores encyst and then germinate and penetrate through stomates. Sporangia are usually inactivated after 5-7 hours in sunlight (Kennelly et al., 2007), so most infections occur in the morning. After infection, the pathogen grows intercellularly, producing haustoria.
Sporangia for secondary infections are produced on sporangiophores that emerge through stomata of infected leaves and other grapevine tissues (e.g. bunches). Production of sporangiophores and sporangia requires 95 to 100% relative humidity and at least 4 hr of darkness at temperatures initially exceeding 13ºC (55ºF). The longevity and productivity of foliar lesions is related with how often they were induced to sporulate. In the absence of an opportunity to sporulate or extremely high temperatures, lesions typically maintained their maximum potential to produce sporangia for at least 22-24 days or even as long as 2-3 months (Kennelly et al., 2007). Caffi et al. (2013) reported the sporangia are produced at a high rate for the first 4 days and then at a lower rate until sporulation stopped. Sporangia are dispersed to new infection sites by rain splash and/or wind; the latter occurs when sporangia are released into the air as the humidity decreases. Zoospores from these sporangia are released in water and are further spread by wind or rain splash.
The incubation period (the time from infection to appearance of new symptoms) varies from 5 to 21 days depending on temperature, target organ of the host, and on ontogenic resistance (Rossi et al., 2013). The incubation period is shortest (~5 days) at mean temperatures from 20-25ºC (68-77ºF). At mean temperatures of 12ºC (54ºF) or lower, the incubation period is 14 days or longer. The latent period on leaves (the time from infection to first production of sporangia) is one day less than the incubation period, given favorable conditions, i.e. sporulation may occur one day prior to the appearance of oilspots.
Sexual reproduction occurs towards the end of the season. The resulting oospores are thick-walled and serve as survival spores.
Disease Management
Cultural practices
When establishing vineyards the location, drainage, type of irrigation and trellising system should all be selected to reduce the risk of disease. Because moisture favors the development of downy mildew, grapevines should be established in well-drained sites with good air movement. Grapevines should be planted in rows that take advantage of natural patterns of air movement to help minimize leaf wetness. Few cultural management options are available to control downy mildew in established vineyards. Trellising systems and pruning can be used to manage the leaf canopy to minimize leaf wetness. Avoid increasing humidity and leaf wetness at night to mitigate secondary infection. Where possible, the use of overhead irrigation should be avoided or scheduled so that leaves will dry quickly. Reducing leaf litter and pruning may reduce the amount of overwintering inoculum.
Genetic resistance
All cultivars of
Vitis vinifera (the Eurasian species) are considered susceptible to downy mildew, although cultivars such as Chardonnay, Pinot Noir, and Sultana are considered more susceptible than Cabernet Sauvignon and Semillon. Several North American species show resistance to downy mildew (e.g.
V. labrusca and
V. rotundifolia), although the
V. labrusca cultivars Niagara and Catawba are highly susceptible. Interspecific hybrids of
V. vinifera and the North American species have yielded cultivars with good wine-grape qualities and greater resistance to downy mildew. However, wine consumers prefer the known varietal wines over the less-known and sometimes fruitier hybrids. There are several active research programs to genetically modify
V. vinifera cultivars to include disease resistance.
Chemical control
Both pre-infection (protective) and post-infection (systemic or penetrant) fungicides are widely used for the control of downy mildew (Figure 9). Pre-infection chemicals are applied prior to, but as close as possible, to an infection event. If used in a regular spray schedule, their best use is in the period of greatest host susceptibility, between shoot length of 10 cm (4 in.) and pea-sized berries. Alternatively, pre-infection chemicals may be used irregularly by spraying as close as possible prior to forecast weather events favorable for
P. viticola infection. The frequency of the sprays will be determined by the prevailing weather conditions. Pre-infection fungicides include the copper-based fungicides, such as Bordeaux mixture (see section on Significance) and the dithiocarbamates. There have been no reports of resistance to these types of fungicides in the pathogen.

Figure 9 |
Post-infection fungicides are more costly than pre-infection fungicides and are best used sparingly. For optimal use, they should be applied as soon as possible after an infection event and prior to the appearance of oilspots. These fungicides are best used in conjunction with a forecasting program, which assesses the likelihood of infection from canopy micro-climate data (Figure 10). A good number of prediction models have been established, such as Australian D-Model (Magarey et al., 1991), DMCast model in the US (Park et al.,1997), EPI model (Stryzik, 1983) and POM model in France (Tran Manh Sung et al., 1990), and complex mechanistic UCSC model in Italy (Rossi et al., 2008). However, only limited models have been tested independently by their developers or have been integrated into more complex advisory systems (Gessler et al., 2011). Some post-infection fungicides are less effective when applied to oilspots, although these fungicides may have the capacity either to kill the pathogen active in oilspots or to significantly reduce its sporulation potential. Currently used post-infection fungicides include phosphonate (e.g. fosetyl-aluminum), phenylamides (e.g. melalaxyl), QoI (e.g. azoxystrobin), and Carboxylic acid amides (CAA; e.g. mandipropamid). Depending on the regulation of each state, some of these chemistries are recommended for controlling grape downy mildew in the US. However, fungicide resistance to some of above mentioned chemicals, such as QoI or CAA fungicides, have also been reported in Australia, Japan, France, and the US (FRAC, 2013). Although some new chemistries are available now, care still need to be taken to prevent the development of fungicide resistance.

Figure 10 |
The disease should be monitored if a post-infection application strategy is used. To scout a vineyard for the presence of downy mildew (Figure 11), the scout should walk slowly along the vines looking for oilspots on at least 200 vines. More than 2 oilspots per 50 vines would be considered a risk to the vineyard.

Figure 11 |
Significance
Grapevines are one of the most widely grown fruit crops in the world with significant plantings in Europe, North and South America, South Africa and Australasia. Grapes are used in the production of wine, brandy, or non-fermented drinks and are eaten fresh or dried as raisins.
In an effort to control a root-feeding aphid called
Phylloxera, resistant grape rootstocks were introduced to Europe from North America in 1878. At the same time, the downy mildew pathogen was introduced into France, probably as oospores on the imported rootstocks, and spread widely throughout Europe. In 1885, P.M.A. Millardet (Figure 12) first used Bordeaux mixture to control downy mildew. It is said that a farmer had applied this mixture of copper sulfate and lime to produce a chemical residue on grapes along the roadside to discourage pilfering by passersby. Millardet's extensive experimentation led to the development of Bordeaux mixture as the first widely used chemical to protect plants from fungal infections. His description of this work has been translated into English as a Phytopathological Classic and is cited in the references. A statue of Millardet was erected in a park in Bordeaux to express the gratitude of the French people for his important discovery. Bordeaux mixture remains an important fungicide even today.

Figure 12 |
Downy mildew is a highly destructive disease of grapevines in all grape-growing areas of the world where there is spring and summer rainfall at temperatures above 10º C (50º F). Crop losses in individual years can be 100% if the disease is not controlled during favorable weather. Early infection of young bunches can lead to significant crop loss, whereas, severe leaf infection affects the source-sink relationship in the vine and may lead to defoliation and possible sunburn or lack of fruit ripening. This destruction of leaf tissue may affect sugar accumulation and growth in the subsequent season (Figures 13 and 14). Currently, there are no suitable sources of resistance in commercially acceptable varieties, so fungicides are the primary means of disease control.

Figure 13 |

Figure 14 |
Selected References
Caffi, T., G. Gilardi, M. Monchiero, and V. Rossi. 2013. Production and release of asexual sporangia in
Plasmopara viticola. Phytopathology 103: 64-73.
Carefoot, G.L. and E.R. Sprott.1967. Famine on the Wind. Rand McNally and Co., Chicago.
Coombe, B.G. and P.R. Dry (eds.). 1992. Viticulture Volume 2. Practices. Winetitles. Adelaide, Australia.
FRAC. 2013. FRAC List of plant pathogenic organisms resistant to disease control agents.
http://www.frac.info/docs/default-source/publications/list-of-resistant-plant-pathogens/list-of-resistant-plant-pathogenic-organisms---february-2013.pdf?sfvrsn=4.
Genet, J.L., and O. Vincent.1999. Sensitivity of European
Plasmopara viticola populations to cymoxanil. Pest. Sci. 55:129-136.
Gessler, C., I. Pertot, and M. Perazzolli. 2011.
Plasmopara viticola: a review of knowledge on downy mildew of grapevine and effective disease management. Phytopathologia Mediterranea 50: 3-44.
Hong, C.F. 2016. Release of
Plasmopara viticola zoospore.
https://www.youtube.com/watch?v=BKs8WM01iIk
Hong, C.F. 2016. Time lapse video of sporulation and sporangia germination of
Plasmopara viticola.
https://www.youtube.com/watch?v=qnE91MbZrsg
Hoppmann D. and K.P. Wittich. 1997. Epidemiology-related modelling of the leaf-wetness duration as an alternative to measurements, taking
Plasmopara viticola as an example. Zeit. Pflanzenkrank. Pflanzenschutz (Journal Plant Dis. Prot.) 104:533-544.
Kennelly, M. M., D. M. Gadoury, W. F. Wilcox, P.A. Magarey, and R.C. Seem. 2005. Seasonal development of ontogenic resistance to downy mildew in grape berries and rachises. Phytopathology 95:1445-1452.
Kennelly M.M., D. M. Gadoury, W. F. Wilcox, P. A. Magarey, and R.C. Seem. 2007. Primary Infection, Lesion Productivity, and Survival of Sporangia in the Grapevine Downy Mildew Pathogen
Plasmopara viticola. Phytopathology 97, 512-22.
Kortekamp A. 1997.
Epicoccum nigrum link - a biological control agent of
Plasmopara viticola (Berk. Et Curt.) Berl. Et de-toni. Vitis 36:215-216.
Lalancette, N., L.V. Madden, and M.A. Ellis. 1988. Development of an infection efficiency model of
Plasmopara viticola on American grape based on temperature and duration of leaf wetness. Phytopathology 78:794-800.
Lalancette, N., L.V. Madden, and M.A. Ellis. 1988. A quantitative model for describing the sporulation of
Plasmopara viticola on grape leaves. Phytopathology 78:1316-1321.
Langcake, P. and A. Lovell. 1980. Light and electron microscopical studies of the infection of
Vitis spp. by
Plasmopara viticola, the downy mildew pathogen. Vitis 19:321-337.
Large, E.C. 1940. The Advance of the Fungi. Dover Publications, New York, NY. Reprinted by American Phytopathological Society Press, St. Paul, MN.
Madden, L.V., M.A. Ellis, N. Lanlancette, and L. L. Wilson. 2000. Evaluation of a disease warning system for downy mildew of grapes.
Plant Dis. 84:549-554.
Magarey, P.A., M.F. Wachtel, P.C. Weir, and R.C. Seem.1991. A computer-based simulator for rational management of grapevine downy mildew
Plasmopara viticola. Aust. Plant Prot. Q. 6:29-33.
Millardet, P.M.A. The discovery of Boreaux mixture, 1885. Phytopathological Classic translated into English by F.J. Schneiderhan. American Phytopathological Society Press, St. Paul, MN.
Park, E.W., R.C. Seem, D.M. Gadoury, and R.C. Pearson.1997. DMCAST: A prediction model for grape downy mildew development. Vitic. Enol. Sci. 52:182-189.
Pearson, R.C. and A.C. Goheen. 1994. Compendium of Grape Diseases. American Phytopathological Society Press, St. Paul, MN.
Rossi V, T. Caffi, S. Giosuè, and R. Bugiani. 2008. A mechanistic model simulating primary infections of downy mildew in grapevine. Ecological Modelling 212, 480-91.
Rossi V, T. Caffi, and D. Gobbin. 2013. Contribution of molecular studies to botanical epidemiology and disease modelling: grapevine downy mildew as a case-study. European Journal of Plant Pathology 135, 641-54.
Wong, F.P., H.N. Burr, and W. F. Wilcox. 2000. Heterothallism in
Plasmopara viticola (grapevine downy mildew). Phytopathology 90:S85.
