Ergot of rye
Claviceps purpurea
Rye and ryegrass (principal economic hosts), barley, oats, triticale, wheat, and other cultivated and wild grass species in the subfamily Pooideae, including bentgrass, bluegrass and fescue.
Ergot is a fungal disease caused by fungi of the genus Claviceps. Species in this genus are unique in that they only infect ovaries of the host plants; no other part of the plant is infected. There are approximately 40 species of Claviceps with C. purpurea (Fries ex Fries) Tulasne being the species of greatest concern. Although C. purpurea has a very broad host range, including approximately 400 grass species, the most economically important of these is rye. Although ergot of rye causes yield reductions, the significance of the disease is primarily related to the toxic alkaloids present in the ergots (sclerotia). The alkaloids can cause severe health problems in both humans and animals. Severe poisoning outbreaks as a result of ergot fungi are called ergotism.

Ergot sclerotia protruding from rye heads.
(Courtesy R. A. Kilpatrick—Copyright APS)
Symptoms and Signs
The first obvious sign of ergot infection is appearance of ‘honeydew’, a sticky yellow sugary solution consisting of host sap and conidia (Figure 1) between the affected glumes of the rye. This is secreted by the infected plant ovary, which eventually is replaced by a purplish-black sclerotium, commonly referred to as an ergot (Figure 2). The size of the sclerotium depends on the host plant; it is generally 1 to 5 times larger than the host seed. Thus, the largest ergots (1-5 cm, 0.4-2 inches) are found in large-seeded plants such as cereal rye. The sclerotium consists of a whitish mycelial tissue containing storage cells and a dark pigmented outer cortex that protects the fungal mycelia from desiccation, UV light and other adverse environmental conditions.

Figure 1. Honeydew
stage of ergot of rye. |

Figure 2. Ergot sclerotia
protruding from rye heads. |
Pathogen Biology
Sexual reproduction
Sexual reproduction involves the fusion of female ascogonia and male antheridia, karyogamy to form diploid nuclei, which is followed by meiosis to return to the haploid state. This results in the production of sexual fruiting bodies called perithecia that contain multiple asci, each of which contain eight filiform ascospores that are up to 2 µm in diameter and 60-70 µm long. Ascospores are ejected from the asci and perithecia into the air in an explosive manner (to a height of 7-15 cm) and are disseminated by air currents or by insects. Each sclerotium produces up to one million ascospores. Only those ascospores that land on a host stigma or ovary can cause infection. The stigma of a grass flower is large and featherlike to trap windborne pollen. This same feature traps the airborne ascospores. Ascospores, which are the primary (initial) inoculum, germinate and infect the ovary within 24 h.
Asexual reproduction
A germinated ascospore produces a long filamentous hypha that colonizes the ovary of the host plant flower. As the hyphae grow longer and more numerous, they are called a mycelium. Fungal mycelium produces numerous asexual conidia (secondary inoculum) from palisade conidiophores within a sweet, yellowish, mucilaginous substance called honeydew. This stage is also called the sphacelia or honeydew stage. The honeydew attracts insects to the wind-pollinated flowers. Insects contaminated with conidia may visit healthy flowers where new infections are initiated. Splashing raindrops also aid in the dispersal of the conidia. Conidia from ergot-infected wild grasses, particularly in fence rows, can be the primary inoculum in cereal and grass seed production fields. Over time, hyphae consume the entire ovary and hyphal threads become thicker and interwoven. Meanwhile, conidia and honeydew production ceases. Mutual pressure of the ever-growing hyphae causes production of dense mass of compact tissue called pseudoparenchyma, which eventually develops into a hard, dark colored sclerotium, or ergot.
Disease Cycle and Epidemiology
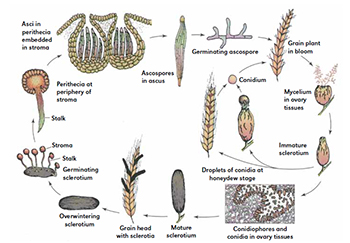 Figure 3. Disease cycle of ergot of small grain cereals and grasses.
Figure 3. Disease cycle of ergot of small grain cereals and grasses.Disease Cycle
The sclerotium (or ergot) is the survival or overwintering structure of C. purpurea. At harvest, a fraction of sclerotia is harvested along with the grain and another fraction falls on the ground. Sclerotia lie dormant until they are exposed to favorable weather conditions. Four to eight weeks of 0 – 10°C temperatures is required for vernalization of the sclerotia before germination. Sclerotia germinate in the spring, just prior to flowering in the cereals and grasses, and give rise to a stroma (stromata plural), formed in mushroom-like fashion on stipes with spherical capitula. It is within these stromata that sexual reproduction occurs (Figure 3).
Epidemiology
Claviceps purpurea is common in temperate climates in which the cold period required for sclerotial germination is met. In warmer climates, such as the southeastern U.S., sclerotia are colonized by other fungi and do not survive well.
Rainfall or high soil moisture is required for stroma formation and ascospore production. In cereal grains and many of the grasses, resistance to infection develops after fertilization. Thus, conditions that delay or interfere with pollination, such as cool, wet weather, can increase the period of susceptibility.
Conidia are an important means of secondary spread. Any transfer of honeydew from infected to healthy flowers can lead to infection. Secondary spread can occur through any means that moves conidia to healthy flowers, including rain splash, insects, head-to-head contact, and moving equipment.
Disease Management
Prevention is the best management strategy, but once ergot is observed in the field, there is not much a farmer can do to control the disease.
Cultural Practices
Cleaning seed: Even though this is labor intensive and expensive, most ergots can be removed from grain by gravity-based cleaning equipment or flotation in brine (e.g., a 20% salt solution).
Planting clean seed: There are no effective seed treatments against ergot, so only ergot-free seed should be planted. Use of certified seed can greatly reduce the risk of planting ergot infected seed.
Ensure uniform stands: Only seed with a good germination percentage should be used. Seed should be sown at a consistent depth, following a balanced fertilizer program and other recommended agronomic practices. Non-uniform stands within a region can result in a prolonged flowering period, which in turn increases the spread of the disease within the field or from one field to another.
Late tillers and side shoots flowering outside the pollination period of main crop are more affected by ergot than the main shoots, especially when the crop stand is thin due to poor growing conditions. This is because they do not receive enough pollen and they still produce honeydew even after the main crop is mature, thus contributing considerably to the secondary spread.
The best crop husbandry practices against ergot are those that ensure a well-developed, well-nourished crop stand. Thus, adequate seed density and fertilization are very important. Adequate copper fertilization is known to play a role in the control of ergot in wheat. Evidence also exists that copper deficiency produces small anthers and enhances pollen sterility. If the flower stays open for long without pollination, it is susceptible to ergot infection by spores of the fungus for a longer time.
Sanitation: Wild, weedy grasses and cereal volunteers that are within or outside the field should be eradicated before heading. These volunteers could be the first source of ergot inoculum from overwintered sclerotia or as a source of honeydew if produced prior to crop flowering.
Crop rotation: As ergots (sclerotia) do not usually survive for more than one year, rotation with non-susceptible host plants is a viable management tactic for annual crops.
Deep plowing: Ascospores from stromata cannot be discharged into the air if sclerotia are buried in the soil through deep plowing. Although deep plowing buries the ergots, many cereal crops are now grown with "no-till" practices in which the new crop is seeded directly into the stubble from the previous crop to reduce soil erosion. Crop rotation is even more important when deep plowing is not practiced.
Harvesting techniques: Ergot is often more severe around the edges of a field, because spores are transported from roadside grasses by wind and active insects. Prior to harvest, fields should be scouted to determine heavily infected areas and the plants in those areas should be harvested separately.
Field burning: Ergot is one of the most important diseases in grass seed production. As many of the grass species are perennial, tillage and crop rotation are not management options.
Post-harvest field burning has been practiced in the northwestern U.S. to manage ergot and other diseases and pests since the 1940s, but environmental concerns have resulted in legislative restrictions to burning.
Chemical Control
Chemicals have been applied to seed or soil to inhibit production of ascospores from sclerotia, but are not economical. Recently, sterol-inhibiting fungicides have been applied at the onset of flowering to prevent infection in Kentucky bluegrass seed production, but such treatments are not usually economical in cereal production.
Genetic Resistance
Interest in genetic resistance to ergot has increased since the 1970s with the creation of male-sterile lines of wheat and barley for hybrid seed production. Wheat and barley are self-pollinated plants, unlike rye which is cross-pollinated. Effective male-sterility systems provide the opportunity for hybrid seed production; however, male-sterile plants flower longer and remain susceptible until they are fertilized.

Figure 4. St. Anthony. Redrawn from a woodcut made in Germany about 1440-50 A.D.
|

Figure 5. St. Anthony accompanied with his
symbol, a pig. From a medieval woodcut. The
sufferer is shown with an amputated foot and
the sacred fire coming from his hand. |
Historical Significance
Ergot word derives from the Latin word articulum (articulation or join) via the Old French argot (cockspur, suggesting the shape of the sclerotium of the fungus, as people in France noted some resemblance between the sclerotia and the spurs on rooster legs). In the European Middle Ages, diseases caused by ergot were referred to as St. Anthony’s fire or holy fire (Figures 4 and 5). In major outbreaks, death rates were reported to be between 10 and 20%. Before this disease was understood, the ergots were ground up along with rye grains and ingested when the flour was used for baking. Its history is unclear because diagnostics were primitive and symptoms could be attributed to a number of diseases. Symptoms also varied depending on which toxins (or alkaloids) were present in the ingested ergots and at what concentration. There are two main clinical toxicity forms of ergotism: gangrenous and convulsive. Common symptoms included strange mental aberrations, hallucinations, a feeling of burning skin or insects crawling under the skin. Some victims developed gangrene due to constriction of blood vessels in the extremities; many victims lost hands and feet (Figure 6). Patients with ergotism were cared for in hospitals dedicated to St. Anthony until their painful and prolonged suffering ceased.

Figure 6. Leg lesions of a calf affected by ergotism. Note the
sharp demarcation between living (top) and dead (bottom) tissue. |
People under the influence of ergot alkaloids may have convulsions, become manic, appear dazed, be unable to speak or have other forms of paralysis or tremors, and suffer from hallucinations and other distorted perceptions. These strange behaviors are similar to those reported for rural peasants during the “Great Fear” at the start of the French Revolution, and those of the women and children charged with witchcraft in 17th Century Europe and the U. S. (Salem, MA, in particular). Because of this and reports that rye was consumed by these populations, it has been proposed that ergotism contributed to these events. However, not everyone agrees and concrete evidence to support the connection is lacking.
Ergots are so commonly associated with rye that they were included in early botanical drawings of the plant species (Figure 7). The discovery of the cause of ergotism in 1670 is attributed to a French physician, Dr. Thuillier. Ergotism could then be reduced by separating the ergots from the healthy grains before milling.
Figure 7. Botanical drawing from early
description of rye showing ergots. |
Thousands of people have died of ergotism, and mortality rates averaged 40% in some documented epidemics in the 1800s. Even after the cause of ergotism was known, many poor people did not have alternative food sources in years when ergot was severe. Many lives have probably been saved by the adoption of the potato, which originated in South America. As the potato became a peasant staple, production of rye and its accompanying ergot disease declined in many areas.
Women suffering from ergotism frequently miscarried, and fertility was generally reduced during outbreaks. When ergotism occurred in lactating women, the ergot alkaloid, ergocryptine, which can inhibit prolactin production and release from the anterior pituitary gland, caused them to stop producing milk.
Historically, ergot was also noted to have effects on parturition and on postpartum hemorrhage. Powdered preparation of ergot called pulvis ad partum (powder of birth) was used by midwives and doctors for many years to accelerate labor, induce abortions and prevent uterine hemorrhage.
At the end of the nineteenth century, ergot was still regarded as a ‘glorious chemical mess’ and people began to use ergot as an important therapeutic agent. The price of ergot, by weight, was 20 times that of rye in the period before the First World War.
Fascination with C. purpurea has resulted in its appearance in various fictional works. Robin Cook based his 1994 novel, Acceptable Risk, on an ergot-like fungus isolated in Salem, Massachusetts. A character in an episode of the television series, X-Files, develops strange behaviors after receiving a tattoo colored with a rye extract.
Current Significance
Modern management practices reduce ergot infections in most cereal crops. Occasionally, animals released into fields for grazing are poisoned by ergot in wild grasses, particularly following prolonged cool, wet weather in the spring. Ergot can also be a problem where cattle producers rely on grass hay for feed during the winter. When fed to cattle, levels of ergot greater than 0.3%, by weight, can cause loss of ear tips and other extremities during very cold weather. Ergot remains a significant problem in grass seed production by reducing yields, creating harvesting problems because of honeydew residues, and causing restrictions on shipment of contaminated seed to other countries. Modern cleaning methods remove ergots from grain before it is milled or used for animal feed, but the process is costly and may leave toxic residues. The legal limit of ergot (by weight) is 0.3% for rye, 0.05% for wheat, and 0.1% for barley, oats, or triticale. If the limit is exceeded, the grain is classified as "ergoty" and its value is reduced. Ergot toxins are not destroyed by baking.
Isolation of ergotamine by Arthur Stoll from Switzerland marks the onset of modern research on ergot alkaloids. Since then, chemists have intensively studied more than 40 alkaloids produced by the fungus, including the infamous hallucinogen, lysergic acid diethylamide (LSD). LSD was first synthesized by the Swiss chemist Albert Hofmann in 1938, while searching for respiratory and circulatory stimulants. Ergot is now deliberately cultivated in the field and laboratory for medicinal purposes. Various compounds have been isolated and modified from ergot for medicinal uses, such as treatment of migraine headaches, post-partum bleeding, neurological and cardiovascular disorders and in modern obstetrics.
Additional Related Fungi
Ergot in Pearl millet: Ergot caused by C. fusiformis is a widespread, destructive and economically important disease of pearl millet (Pennisetum glaucum). It is widespread in Africa and India where pearl millet has been grown for thousands of years. Its importance as a major threat was realized only after the introduction of hybrids based on cytoplasmic male sterility (CMS). This disease has been reported from India, Pakistan and several countries in Africa. C. fusiformis has not been reported on pearl millet in the U.S.
Cream to pink mucilaginous honeydew oozes from infected florets on pearl millet panicles
(Figure 8). The honeydew contains two types of asexual spores (macro- and micro conidia) that lead to secondary spread of the disease. Within 2 weeks, these droplets dry out and hard, dark brown to black sclerotia, which are larger than the seed and have a pointed apex, protrude from the florets in place of grain (Figure 9).
Sclerotia germinate under favorable conditions and produce ascospores that are carried by air currents and infect stigmas of pearl millet before pollination. Honeydew symptoms appear within 4-6 days and fully developed sclerotia are visible within 15-20 days after infection.
Pearl millet ergot sclerotia contain groups of water-soluble alkaloids that are different from those of C. purpurea ergot. Ergotism symptoms caused by pearl millet ergot are also different from those of European classical ergotism. Agroclavme and elymociavine are two groups of alkaloids identified from pearl millet.
 |
 |
|
Figure 8. The “honey-dew” (conidial) stage of Claviceps fusiformis on an inflorescence of pearl millet (Pennisetum typhoides). |
Figure 9. Sclerotia of Claviceps fusiformis protruding from pearl millet head. |
Ergot in Sorghum: Several Claviceps species cause ergot disease in sorghum. In 1995, Claviceps africana was discovered in Brazil, the first report outside Africa and Asia. It rapidly spread worldwide in most sorghum-growing areas including the U.S. It was first detected in the U.S. in Texas in the 1997 growing season, during which this disease spread from the lower Rio Grande Valley to the high plains of Texas and then into Kansas, Nebraska, New Mexico and Georgia. The life cycle is similar to that of C. purpurea with the addition of a second, airborne conidial stage on the surface of the honeydew droplet (Figure 10), in addition to the sticky conidia, which contributes to its rapid spread.
Sorghum ergot produces different range of alkaloids than rye and other millet ergots. Sorghum ergot was considered less toxic to animals but sudden threat of the disease worldwide has led to new studies which concluded the toxic effects of sorghum ergots on lactation, plasma prolactin level and weight gain in animals.

Figure 10. Honeydew stage of sorghum ergot. |
Epichloë spp.
Epichloe spp. belong to the genus of ascomycete fungi and same family as the ergot fungus (Clavicipitaceae). They are known to form endophytic symbiosis with grasses and are ecologically significant through their effect on host plants. For most of their life cycle, Epichloe grow in the intercellular spaces of stems, leaves, inflorescences and seeds of the grass plant without incurring symptoms of disease. Their presence is reported to provide several benefits to their host, including the production of different herbivore deterring alkaloids, increased tolerance to biotic and abiotic stresses; and growth promotion.
Although grass-Epichloe spp. symbioses have been widely recognized to be mutualistic and beneficial to many wild and cultivated grasses, some interactions can be highly variable and sometimes antagonistic. For example; grass choke disease (Figure 11) is caused by some species of Epichloë, which form spore bearing mats (stromata) on tillers and suppress the development of host plant's inflorescence.

Figure 11. Grass choke disease in turf grass with spore
bearing stroma of Epichloë typhina. |
Epichloe spp.-grass endophytic interaction also results in alkaloid production which cause animal diseases such as "fescue toxicosis"," ryegrass staggers", "sleepy grass" and "drunken horse grass". On the other hand, peramine and loline alkaloids are deterrent and/or toxic to insects.
Selected References
Agrios, G. N. 2005. Plant Pathology. 5th ed. Elsevier–Academic Press, Boston.
Bandyopadhyay, R., Frederickson, D. E., McLaren, N.W., Odvody, G. N., and Ryley, M. L. 1998. Ergot: A new disease threat to sorghum in the Americas and Australia. Plant Dis.
82:356-367.
Blaney, B. J., Kopinski, J. S., Magee, M. H., McKenzie, R. A., Blight, G. W., Maryam, R., and Downing, J. A. 2000. Blood prolactin depression in growing pigs fed sorghum ergot (Calviceps africana). Aus. J. Agri. Res. 51:758-791.
Bové, F. J. 1970. The Story of Ergot. S. Karger, New York.
Caporael, L. R. 1976. Ergotism: The Satan loosed in Salem? Science 192:21-26.
Carefoot, G. L., and Sprott, E. R. 1967. Famine on the Wind. Rand McNally, Chicago.
Esser, K., and Düvell, A. 1984. Biotechnological exploitation of the ergot fungus (Claviceps purpurea). Process Biochem. 19:142-149.
Evans, I. R., Maurice, D. C., Penney, D. C., and Solberg, E. D. 1993-1994. Wheat diseases and copper nutrition. Better Crops, pp. 6-8.
Graham, R. D. 1975. Male sterility in wheat plants deficient in copper. Nature 254:514-515.
Guerre, P. 2015. Ergot alkaloids produced by endophytic fungi of the genus Epichloe. Toxins 7:773-790.
Hudler, G. W. 1998. Magical Mushrooms, Mischievous Molds. Princeton University Press, Princeton, NJ.
Johnston, W. J., Golob, C. T., Sitton, J. W., and Schultz, T. R. 1996. Effect of temperature and postharvest field burning of Kentucky bluegrass on germination of sclerotia of Claviceps purpurea. Plant Dis. 80:766-768.
Kirby, H. W. 1998. Ergot of cereals and grasses. University of Illinois Extension. RPD No. 107. https://ipm.illinois.edu/diseases/rpds/107.pdf
Kopinski, J. S., Blaney, B. J., Murray, S. A., and Downing, J. A. 2008. Effect of feeding sorghum ergot (Claviceps africana) to sows during mid lactation on plasma prolactin and litter performance. J. Anim. Physiol. Anim. Nutr. 92:554-561.
Kren, V., and Cvak, L., eds. 1999. Ergot: The Genus Claviceps. Harwood Academic, Amsterdam, The Netherlands.
Kuldau, G., and Bacon, C. 2008. Clavicipitaceous endophytes: Their ability to enhance resistance of grasses to multiple stresses. Biol. Control. 46:57-71.
Lee, M. R. 2009. The history of ergot of rye (Claviceps purpurea) I: From antiquity to 1900. J. R. Coll. Physicians Edinb. 39:179-184.
Lorenz, K. 1979. Ergot on cereal grains. CRC Crit. Rev. Food Sci. Nutr. 11:311-354.
Mathre, D. E., ed. 1997. Compendium of Barley Diseases. 2nd ed. American Phytopathological Society, St. Paul, MN.
Matossian, M. K. 1989. Poisons of the Past. Yale University Press, New Haven, CT.
Miedaner, T., and Geiger, H. H. 2015. Biology, genetics, and management of ergot (Claviceps spp.) in rye, sorghum, and pearl millet. Toxins 7:659-678.
Odvody, G., Bandyopadhyay, R., Frederiksen, R. A., Isakeit, T., Frederickson, D., Kaufman, H., Dahlberg, J., Velasquez, R. and Torres, H. 1998. Sorghum ergot goes global in less than three years. APS Features. doi:10.1094/APSFeature-1998-06.
Puranik, S. B., and Mathre, D. E. 1971. Biology and control of ergot on male sterile wheat and barley. Phytopathology 61:1075-1080.
Rehacek, Z., and Sajdl, P. 1993. Ergot Alkaloids: Chemistry, Biological Effects, Biotechnology. Elsevier, New York.
Schardl, C. L., and Phillips, T. D. 1997. Protective grass endophytes: Where are they from and where are they going? Plant Dis. 81:430-438.
Schlegel, R. H. J. 2014. Rye: Genetics, Breeding, and Cultivation. CRC Press–Taylor & Francis, Boca Raton, FL.
Schultz, T. R., Johnston, W. J., Golob, C. T., and Maguire, J. D. 1993. Control of ergot in Kentucky bluegrass seed production using fungicides. Plant Dis. 77:685-687.
Spanos, N., and Gottlieb, J. 1976. Ergotism and the Salem Village Witch Trials. Science. 194:1390-1394.
Thakur, R. P., and King, S. B. 1988. Ergot disease of pearl millet. Information Bull. No. 24. International Crops Research Institute for the Semi-Arid Tropics, Patancheru, India.
Tudzynski, P., and Scheffer, J. 2004. Claviceps purpurea: Molecular aspects of a unique pathogenic lifestyle. Mol. Plant Pathol. 5:377-388.
Uppala, S., Wu, B. M., and Alderman, S. C. 2016. Effects of temperature and duration of preconditioning cold treatment on sclerotial germination of Claviceps purpurea. Plant Dis. 100:2080-2086.
Wegulo, S. N., and Carlson, M. P. 2011. Ergot of small grain cereals and grasses and its health effects on humans and livestock. University of Nebraska-Lincoln Extension. EC 1880. http://extensionpubs.unl.edu/publication/9000016368209/ergot-of-small-grain-cereals-and-grasses-and-its-health-effects-on-humans-and-livestock/
Wood, G., and Coley-Smith, J. R. 1982. Epidemiology of ergot disease (Claviceps purpurea) in open-flowering male sterile cereals. Ann. Appl. Biol. 100:73-82.
