Updated 2018.
Late blight of potato and tomato
Phytophthora infestans
Potato, tomato (economically important hosts)
Authors
Jean Ristaino, NC State University
Gail L. Schumann, University of Massachusetts, Amherst
Cleora J. D'Arcy, University of Illinois

Figure 1. A potato field in eastern NC with late blight.
Late blight is the disease that caused the Irish potato famine of the 1840s (Figure 1). The pathogen was first described by M. J. Berkeley (Figure 2A) and subsequently named Phytophthora infestans by Anton de Bary (Figure 2B) in the 1870’s (Berkeley, 1846; de Bary, 1876). de Bary's (Figure 2B) (the "father of plant pathology") conclusive studies convinced the scientific community that the white sporulation of P. infestans on infected potato plants was the causal agent of the disease and not the result of spontaneous generation from the decaying vegetation or the wrath of God. Thus, late blight signifies the official beginning of the science of plant pathology. These early studies also contributed to Louis Pasteur's germ theory which was proposed 15 years later.
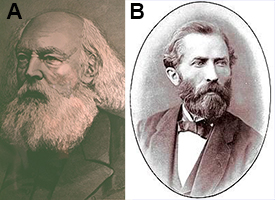
Figure 2. M. J. Berkeley and Anton de Bary. |
Symptoms and Signs
Potato stems and leaves
Late blight of potato is identified by blackish/brown lesions on leaves and stems (Figures 3,4) that may be small at first and appear water-soaked or have chlorotic borders but expand rapidly and the entire leaf becomes become necrotic. In humid conditions, P. infestans produces sporangia and sporangiophores on the surface of infected tissue and the resulting white sporulation can be seen at the margins of lesions on abaxial (lower) surfaces of leaves (Figures 5). As many lesions accumulate, the entire plant can be destroyed in a matter of days after the first lesions are observed if the appropriate fungicide applications are not used (Figure 6).

Figure 3. A late blight lesion on a potato leaf. |

Figure 4. Stem lesion on potato. |

Figure 5. Lesion on potato leaf with
white sporulation. |

Figure 6. Potato plants with severe
symptoms of late blight. |
Potato tubers
Potato tubers can become infected in the field when sporangia are washed from lesions on the foliage and enter into the soil. Infections generally begin in tuber cracks, eyes or lenticels. Infected tuber tissues (Figures 7,8) are copper brown, reddish or purplish in color. Sporulation (Figure 9) may occur on the surface of infected tubers in storage or on discarded cull piles. Infected tubers are often invaded by soft rot bacteria which rapidly convert adjoining healthy potatoes into a smelly, rotten mass that must be discarded (Figure 10).

Figure 7. External symptoms on
infected tuber. |

Figure 8. Internal discoloration of
infected tuber. |
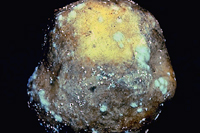
Figure 9. Infected tuber with
surface sporulation. |

Figure 10. Cull pile of
tubers. |
Tomato stems and leaves
Tomato plants are also susceptible to late blight, and the foliar symptoms are similar to those on potato. Like potato, infected tomato plants (Figure 11) may be rapidly infected and destroyed by P. infestans. White sporulation (sporangia and sporangiophores) (Figure 12) may be visible in humid weather. The pathogen may also spread in stems and in tomato transplants and (Figure 13). A large epidemic in 2009 in the US was caused by spread of the pathogen on infected tomato transplants (Hu et al., 2011).

Figure 11. Infected tomatoes
with late blight. |

Figure 12. Infected tomato
leaves. |
Tomato fruit
Symptoms of late blight infections on tomato fruit is similar to other Phytophthora diseases and dark brown, firm lesions (Figure 14) occur that may enlarge and destroy the entire tomato fruit. Late blight lesions on tomato fruit are often followed by soft rot and disintegration as described for potato tubers. Infected fruit that are culled may also be a source inoculum if sporulation and aerial dispersal occurs.

Figure 13. Tomato transplant with stem lesion. |

Figure 14. Infected tomato fruit. |
Pathogen Biology
Phytophthora infestans was first named Botrytis infestans by M. J. Berkeley in the 1840’s. In 1876, the pathogen was renamed by Anton de Bary to Phytophthora infestans (de Bary, 1876). The name is derived from the Greek: Phyto = plant, phthora = destroyer. Phytophthora infestans is a member of the oomycetes, a group of organisms sometimes referred to as the "water molds". Oomycetes are not true fungi but are more closely related to brown algae. The mycelium is hyaline and coenocytic (few septa), and the nuclei are diploid. Most fungi are haploid. The diploid life cycle of Phytophthora was first described by Eva Sansome, a plant geneticist (Ristaino et al., 2007). The family name for this group of organisms is Peronosporaceae which have been assigned to the Kingdom Stramenopila of the eukaryotes. Oomycetes are no longer considered members of the Kingdom Fungi although they share many biological, ecological, and epidemiological characteristics with fungal plant pathogens).
Asexual reproduction
Phytophthora infestans produces sporangia or sac-like structures on sporangiophores (Figure 15). The sporangiophores are compound and indeterminate and (i.e., they grow and produce sporangia continuously). These stalk-like structures aid in air dispersal of the sporangia. Phytophthora infestans is one of the few species in the genus Phytophthora adapted to air dispersal. Sporangia may be dispersed to neighboring fields, but do not generally survive long-distance travel because of desiccation and exposure to solar radiation. The disease is considered a community disease since it can spread from field to field if fields are left untreated.
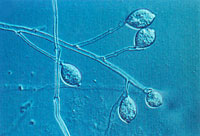
Figure 15. Compound sporangiophore
and sporangia. |
In cool, wet conditions, zoospores will form and emerge (Figure 16) from the sporangia after about two hours. In warmer conditions, sporangia may function as a single spore and germinate directly (Figure 17). Zoospores are biflagellate (have two flagella) (Figure 18), with one tinsel flagellum directed anteriorly and one whiplash flagellum directed posteriorly. After swimming on the surface of the host plant surface, zoospores encyst and infect the plant.
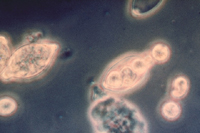
Figure 16. Zoospores emerging
from a zoosporangium. |
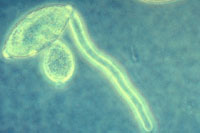
Figure 17. Germinating sporangia.
|
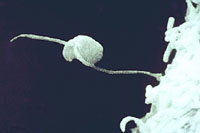
Figure 18. Zoopsore with
biflagellate flagellum. |
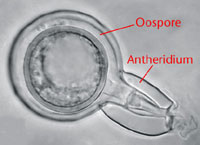
Figure 19. Sexual oospore.
|
Sexual reproduction
If both mating types (A1 and A2) come into contact with one another, sexual reproduction may occur. Meiosis occurs in the gametangia and then a nucleus from the antheridium enters the oogonium. Following karyogamy (the fusion of two nuclei) in the oogonium, a thick-walled, diploid oospore (Figure 19) is formed. Sexual reproduction was first reported in Mexico and the mating types were described there (Galindo and Gallegly, 1958). The pathogen reproduces sexually in the Netherlands and parts of Scandinavia.
Disease Cycle and Epidemiology
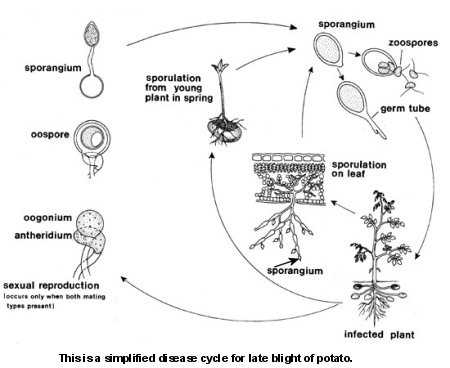 Figure 20. disease cycle.
Figure 20. disease cycle. Disease Cycle
In the absence of the oospore stage, P. infestans survives between potato crops as mycelium in infected tubers or tomato fruit. If infected tubers are left behind at harvest or dumped at the edges of fields (Figure 10), sporangia may be produced on the infected tubers or new volunteer sprouts that appear the following spring. Air currents carry sporangia to healthy potato foliage. Sometimes seed potatoes can become infected and stem lesions occur that can kill the plant (Figure 21); freshly cut seed tuber surfaces are especially susceptible to infections from airborne spores in contaminated storage facilities. If infected seed are planted, local infection can occur. The pathogen spreads by movement in infected tuber tissues and asexual reproduction of clonal lineages is common (see Disease Cycle) (Figure 20). An animated video is available to visualize the disease cycle (See Verreet and Klink, 2010) in the teaching tools section of APS PRESS.

Figure 21. Potato seedling
with stem lesions |
In the presence of water and at cooler temperatures, sporangia germinate indirectly (Figure 16) by the production and release of zoospores (Figure 21). At warmer temperatures, the sporangia germinate directly (Figure 17) by the production of a germ tube. Several days after infection, new sporangia are produced on sporangiophores (Figure 20), which emerge from stomata. The deciduous sporangia may be dispersed by wind or water to new parts of the same potato plant or new plants. Sporangia may also be washed through the soil to infect tubers. If both mating types come into contact with each other, thick-walled oospores (Figure 19) may be produced to persist in soil or plant tissues. In areas where asexual reproduction occurs, oospores usually germinate by producing a sporangium (Figure 22).
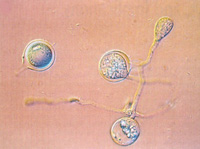
Figure 22. Oospores of P. infestans |
Epidemiology
Temperature and moisture are the most important environmental factors affecting late blight development. Sporangia are formed on the lower leaf surfaces (Figure 5) and infected stems (Figure 4) when relative humidity is < 90%. Sporulation can occur from 3-26°C (37-79°F), but the optimum range is 18-22°C (64-72°F). Sporangia germinate directly via a germ tube at 21-26°C (70-79°F). Below 18° C (65°F), sporangia produce 6 to 8 zoospores which require water for swimming.
Each zoospore is capable of initiating an infection, which explains why disease is more severe in cool, wet conditions. Cool nights, warm days, and extended wet conditions from rain and fog can result in late blight epidemics in which entire potato fields are destroyed in less than two weeks. The pathogen can sporulate on infected tubers in poorly controlled storage areas (Figure 9) where conditions are too humid. Condensation produces water droplets on the surface of infected tubers which may then cause the pathogen to produce sporangia and contaminate neighboring tubers, leading to destruction of the entire pile by soft rot bacteria.
Disease Management
Preplanting management options
Cultivars: No potato cultivar is immune to all lineages of P. infestans, but some cultivars are more resistant than others (Figure 23). If the climate in which the potatoes are grown is relatively dry, even low levels of resistance may significantly reduce disease severity. Likewise improved host resistance can be combined with timely foliar fungicide sprays to enhance effective disease management. Transgenic potatoes have been developed that were modified with genes from wild Solanum species and these transgenic potatoes require fewer fungicide applications and are more environmentally friendly than spraying weekly.

Figure 23. Late blight
resistant variety |

Figure 24. Field bordered by trees
|
Site selection: Good drainage and good air movement will help reduce moisture levels in the crop canopy. Fields bordered by trees (Figure 24) and dense vegetation should be avoided. The shape of the field may affect the ease and frequency of fungicide applications.
Crop rotation: Rotations of two to three years to non-host crops are recommended to control late blight. Besides potato and tomato, several weeds and ornamental plants in the Solanaceae family are known to be susceptible to late blight including nightshades. If oospore production becomes widespread, rotation plans may need to be modified to accommodate this new source of inoculum. The pathogen survives in infected tubers which decay relatively quickly, but oospores may survive in soil for many years (Stevenson, 1993).
Elimination of overwintering inoculum: In the absence of oospores, tubers infected during the previous season are the most important source of initial inoculum. Surviving tubers may be found in cull piles (Figure 10) and tubers left in the field at harvest. Culled potatoes should be left on the soil surface to freeze, trucked to a landfill, or buried at least 1 m (3 feet) deep. In Europe, laws requiring tarping of cull piles have been made to prevent spread of the disease. They also may be fed to livestock provided steps are taken to secure them during transport and dump them only on impervious surfaces not adjacent to susceptible crops. Volunteer plants in the spring should be destroyed to minimize initial inoculum.

Figure 25. Potato sorting for certification. |
Planting of pathogen-free tubers: Only certified seed tubers (Figure 25) should be planted. However, even certified seed tubers may be allowed to have up to 1% incidence of late blight. That is enough inoculuim to cause an outbreak. Diagnostic methods including PCR, real time PCR and LAMP assays have been developed that can be used to confirm the presence of P. infestans in infected tubers. Fungicide treatments are available for protecting freshly cut seed tuber surfaces.
Management in established potato fields
Hilling: Soil can be tilled around plants at the base by hilling after emergence of the young potato plants. Hilling helps in early weed control and minimizes tuber infections from sporangia that wash off the leaves of infected plants into the soil.
Irrigation: It is important to minimize the time that leaves are wet to help prevent foliar infection (Figure 26). Irrigation should be timed so that length of the night dew period is not extended- i.e., no late afternoon, early evening, or morning irrigation in order to allow plants time for drying. Excessive irrigation can also wash some of the "hilled" soil away from the base of the plants, exposing tubers to greater potential infection.

Figure 26.
Irrigation of a potato field. |
Fertilization: Excessive nitrogen fertilization increases canopy growth and cover, delays maturity and may reduce yield. Delayed maturity results in more foliage exposed to potential infection for a longer time, thus increasing the likelihood that tubers may become infected and increases the risk of late blight.
Scouting for disease: Field scouting alerts growers that the potential for a severe disease outbreak exists. Disease tends to occur where moisture is likely to persist (low areas, near hedges and trees) and where fungicide applications may be difficult because of obstructions (corners, trees, utility poles). Infected plants should be burned or plowed under if found in an isolated "hot spot."
Late blight is considered a community disease since sporangia from one field may spread to adjacent fields left untreated posing a threat to neighboring growers. Thus, timely fungicide applications, cultural practices and host resistance are important to manage disease. The USAblight project (USAblight.org) was funded by USDA AFRI in 2011 and provides a set of tools for growers to use to avoid late blight (Figure 27). The disease alert and forecasting system includes a reporting system that allows growers to report the disease in their county, submit a sample for genotyping and fungicide testing, and a map is available so growers can visualize where outbreaks have occurred and receive alerts of late blight nearby (Figure 27).
Current season temperature, relative humidity and rainfall data are used to predict disease outbreaks, based on historical patterns. Decision support tools have been developed for late blight including Blight Pro and this tool is incorporated into USABlight. The tools recommend application of fungicides when the forecast indicates disease development is likely. Forecasting systems for late blight are based on temperature and relative humidity and include the Hyre system, the Wallin system, and BLITECAST which incorporates both systems. The decision support systems are tied into local weather stations and thus advice is tailored for regional forecasts.
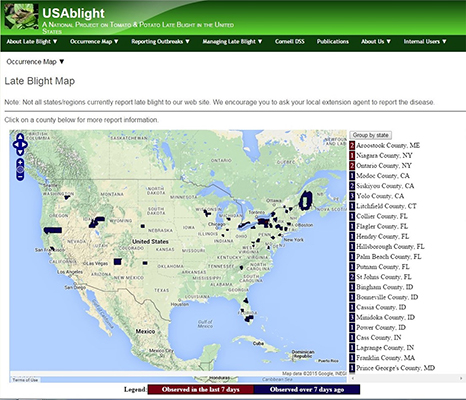
Figure 27. USABlight disease alert map.
|

Figure 28. Applications for late blight on tomato. |
In the US a series of clonal lineages of P. infestans have occurred and the frequency of recent lineages cab be found on USABlight (Fry et al., 2015). Lineages such as US-1 once dominant in the mid 20th century and were recorded as early as 1931 in the US have been displaced (Fry et al., 2013; Hu et al., 2011; Saville et al. 2016). Some lineages cause disease more on potato (US-8, US-14, US-24) while others infect both potato and tomatoes (US-23). A shift in genotypes has occurred between 2009 and 2017 in the US from the mefenoxam sensitive US-22 lineage to the mefenoxam sensitive US-23 lineage (Fry et al., 2013).
Fungicide applications: Fungicide applications (Figure 28) are an important means of late blight management, particularly in humid areas. Contact fungicides are effective and have not resulted in pathogen resistance after many years of use. They coat the leaves to prevent infection, but cannot stop infections once they occur. Therefore, they must be applied before plants are exposed to spores. Systemic fungicides can offer some post-infection control.
Resistance to metalaxyl/mefenoxam was reported in strain of P. infestans in the early 1990s, and some growers lost entire potato crops to disease (Figure 29). The US-8 and US-11 lineages are mefenoxam resistant but current lineages are sensitive to the fungicide (Saville et al., 2015). Systemic fungicides, such as dimethomorph, cymoxanil, fluopicolide and propamacarb have been registered for late blight control. In addition, growers have been encouraged to rotate fungicides and concentrate on the prophylactic use of contact fungicides. Some growers routinely use contact fungicides early in the season, but then rely on forecasting systems after plants mature and the canopy is established.

Figure 29. Potato field lost to late blight.
|

Figure 30. Figure 30 Flame killing potato
vines before harvest. |
Management at harvest and in storage
At the time of harvest, it is possible that some late blight infections are present in the foliage. To prevent inoculation of the tubers during harvesting, foliage needs to be destroyed so that no green tissue remains. In the past, vines were killed with flames (Figure 30). Herbicides are currently used to assure that plants have died completely before harvest. Infected tubers should be removed before going into storage, and disposed of properly. Blighted tubers do not generally store well under typical storage conditions.
Historical Significance
The late blight epidemics of the 1840s triggered the Irish potato famine, but the history of the potato as a food crop is much older. The potato (Solanum tuberosum) originated in the highlands of the Andes in the Lake Titicaca area between Bolivia and Peru, and in Chile, where native people selected hundreds of different cultivars for centuries (Figure 31). Late blight epidemics were first described around the ports of NY and Philadelphia in 1843, and two years later in Europe in 1845. After the introduction of P. infestans potato crops failed for a number of years during the cool and rainy "hungry '40s." Severe epidemics also occurred in the 1870’s.

Figure 31. Chilean woman selling
potatoes in the market. |

Figure 32. An Irish farmer uncovering
rotted potatoes. |
Although poor people who were dependent on potatoes for food suffered in many areas, the disaster was greatest in Ireland where a single adult male consumer 9-11 pounds of potato daily (Figure 32). One and one-half million people starved and a similar number emigrated during the famine, resulting in a large Irish diaspora in many parts of North America (Figure 33). As with many famines, politics enhanced the suffering. Many Irish peasants grew cereal crops to pay their rent. Although the grain was harvested, it could not be eaten, and was exported to the English landlords throughout the famine years.

Figure 33. Famine Memorial Harvard Square. |
Where and how the plant and the pathogen first came together has been a subject of much investigation and debate, but historical records suggest the pathogen was present in the Andean region for a long time and recognized by native people (Berkeley, 1846; Bourke, 1964). Potatoes were shipped from South America during this time period due to dry rot of potatoes that led to a deterioration of seed. Mexico is recognized as a center of diversity of the pathogen and the sexual state of the pathogen was first reported there (Gallegly and Galindo, 1958). Twentieth century outbreaks in the US have been caused by migration of the pathogen from Mexico (Fry et al., 2015). Recent work with genome sequencing of many historic samples that contain the pathogen has identified the lineage that caused the 19th century outbreaks and provided evidence suggesting that ancestral home of P. infestans may lie in the Andean region of Ecuador, Peru and Colombia (Martin et al., 2016, Saville et a, 2016).
Lesson Plans and Teaching Tools
Hite, Rebecca and J.B. Ristaino. Online lesson plans “CSI Dublin: Hunt for the Potato Killer” developed by High School teacher
D'Arcy, C.J. and D.M. Eastburn. 2000. Late blight. Plants, Pathogens, and People.
Eastburn, D.M. and C.J. d’Arcy 1996. Late Blight and the Irish Potato Famine DVS. APS Press.
Verreet, J. and Klink, H. 2010. Potato Blight- The disease cycle of Phytophthora video - DVD Rom. APS Press. https://my.apsnet.org/ItemDetail?iProductCode=44235
Selected References
Berkeley M. J.1846. Observations, botanical and physiological on the potato murain. J Hort Soc London 1: 9-34.
Bourke, P. M. A. 1964.Emergence of potato blight, 1843-46. Nature 203: 805–808.
DeBary A. 1876. Researches into the nature of the potato-fungus - Phytophthora infestans. Journal of the Royal Agricultural Society12:239-268.
Fry, W. E., McGrath, M. T., Seaman, A., Zitter, T. A., McLeod, A., Danies, G., Small, I. M., Myers, K., Everts, K., Gevens, A. J., Gugino, B., Johnson, S. B., Judelson, H., Ristaino, J. B., Roberts, P., B. Secor, G., Seebold, K. W., Jr., Snover-Clift, K. L., Wyenandt, A., Grunwald, N. J., Smart, C. D. 2013. The 2009 Late Blight Pandemic in Eastern USA- causes and results. Plant Dis. 96: 296-306.
Fry, W. E, Birch, P.R. J., Judelson, H. S., Grunwald, N. J., Danies, G., Everts, K. L., Gevens, A. J., Gugino, B. K., Johnson, D. A., Johnson, S. B., McGrath, M. T., . Myers, K. L., Ristaino, J. B., Roberts, P. D., Secor, G., Smart, C. D. 2015. Five reasons to consider Phytophthora infestans a reemerging pathogen. Phytopathology 105: 966-981.
Gallegly, M. and Galindo, J. 1958. Mating types and oospores of Phytophthora infestans in nature in Mexico. Phytopathology 48:274.
Hu, C. H., Perez, F. G., Donahoo, R., McLeod, A., Myers, K., Ivors, K., Secor, G., Roberts, P. D., Fry, W. E., Deahl, K. L., and Ristaino. J. B. 2012. Recent genotypes of Phytophthora infestans in eastern USA reveal clonal populations and reappearance of mefenoxam sensitivity. Plant Dis. 96: 1323-1330.
Martin, M.D., Vieira, F.G, Ho, S.Y.W., Wales, N., Schubert, M., Seguin-Orlando. A. et al. 2016. Genomic characterization of a South American Phytophthora hybrid mandates reassessment of the geographic origins of Phytophthora infestans. Mol Biol Evol. 33: 478–491.
Ristaino, J. 2007. Pioneering Women in Plant Pathology. American Phytopathological Society, APS Press. (http://www.shopapspress.org/piwoinplpa.html) .
Saville, A., Graham, K., Grünwald, N., Myers, K., Fry, W., and Ristaino, J. B. 2015. Fungicide sensitivity of US genotypes of Phytophthora infestans (Mont.) de Bary to six oomycete-targeted compounds. Plant Dis. 99:659-666.
Saville, A., Martin, M, Gilbert, T. and Ristaino, J. 2016. Historic late blight outbreaks caused by a widespread dominant lineage of Phytophthora infestans (Mont.) de Bary Plos One; https://dx.doi.org/10.1371/journal.pone.0168381
Stevenson, W.R. 1993. Management of early blight and late blight. Pages 141-147 in: Potato Health Management. R.C. Rowe, ed. American Phytopathological Society Press, St. Paul, MN.
