DISEASE: Mummy Berry
PATHOGEN: Monilinia vaccinii-corymbosi
HOSTS: Wild species and cultivated blueberries (Vaccinium spp.)

Mummy berry, caused by the fungal pathogen Monilinia vaccinii-corymbosi, is a disease of high economic concern in areas of blueberry production from the southern United States to Canada. The disease results in the replacement of blueberry fruit with a fungal pseudosclerotium causing substantial crop loss. The fungus is an excellent example of floral mimicry by a vector-dependent plant pathogen through exploitation of host pollinators such as bees, moths and flies.
Symptoms and Signs
Symptoms
Following primary infection, the earliest symptom is generally wilting of leaves; newly formed pinkish leaves will often turn a rosy brown and sometimes form a shepherd’s crook or curl; severe wilting of vegetative and floral shoots, followed by a brown discoloration or blight, may occur in some years but not all. Within 72 h, the whole shoot, including any flower buds, will collapse (Figure 1). The highest incidence of infected shoots occurs on the lower canopy, which overhangs moist soil and developing apothecia.

Figure 1
During secondary infection, individual flowers are colonized. Subsequently, blueberry fruit initially appears healthy, but begins to discolor as the disease develops. These previously green berries become pinkish-purple and wrinkled, while healthy blueberries ripen and turn a dark bluish-purple (Figure 2). In addition, infected berries at first appear swollen, then shrivel to about three-fourths of the normal fruit size and drop to the ground.

Figure 2
Signs
From late summer until early spring, fungal overwintering structures called pseudosclerotia (also called “mummies”) rest on the blueberry orchard floor. Pseudosclerotia are hardened masses of fungal hyphae that resist decomposition and allow the fungus to overwinter. Successfully overwintered pseudosclerotia are most commonly found beneath fallen blueberry leaves, moss, or other plant debris. At floral bud break, the pseudosclerotia produce fruiting bodies called apothecia (Figure 3), which discharge sexual spores (ascospores) and cause primary infection. Asci, ascospores, and paraphyses (packing tissue between asci) are hyaline (light-colored) and the ascospores are lemon-shaped.

Figure 3
Following primary infection, secondary asexual spores, called conidia, are produced. Conidia are borne in chains, which can be branched or un-branched. They are also hyaline in color, but when clustered together, take on a grayish appearance which can be observed in the form of a grayish mantle on infected plant parts (Figure 4). There is also a distinctive sweetish odor that is characteristic of many Monilinia species. The conidial mat secretes sugars and reflects UV light which is thought to attract pollinators. The conidia, mixed with sticky sugars, adhere to various bee, moth, and fly species and are vectored to healthy blueberry flowers, where they germinate to cause secondary infection of the fruit.

Figure 4
Once infected, if a developing blueberry fruit is cut open, mats of fungal hyphae (mycelium) can be observed as they invade the fruit locules (Figure 5). This mycelium will continue to colonize the host tissue until it forms a pseudosclerotium, at which point the host tissues will shrivel and the fruit will drop to the ground. This mass of mycelium is soft and cream-colored early in development, but as it grows, the mycelium hardens and becomes a tan color. Late summer pseudosclerotial maturity can be visually assessed based on color. This is typically observed as the surrounding host epidermis is sloughed off. Tan or brown pseudosclerotia represent the most juvenile pseudosclerotial form, followed by grey pseudosclerotia, and mature pseudosclerotia are black.

Figure 5
Pathogen Biology
M. vaccinii-corymbosi was originally described by Longyear in 1901 occurring on wild Vaccinium in Michigan. He classified the fungus as Sclerotinia vaccinii Wor. In 1908, Reade differentiated the pathogen based on its host range and named the fungus S. vaccinii-corymbosi. It wasn’t until 1928, when the new genus Monilinia was established by Edwin E. Honey and the fungus became M. vaccinii-corymbosi (Reade) Honey.
Sexual Reproduction
Historically, there has been a lack of consensus in the scientific community as to whether the “mummy” of M. vaccinii-corymbosi is a sclerotium or a pseudosclerotium. A sclerotium is defined as, “a firm, frequently rounded, mass of hyphae with or without the addition of host tissue or soil, normally having no spores in or on it, and may give rise to a fruiting body.” While a pseudosclerotium is defined as, “a compacted mass of intermixed substratum (soil, stones, etc.) held together by mycelium.” Honey originally described the overwintering structure of M. vaccinii-corymbosi as a pseudosclerotium. However, Milholland later sought to reclassify the structure based on the lack of incorporation of host tissues. According to Milholland, the pseudosclerotium was solely composed of filamentous hyphae and an outer rind of host epidermis cells.
After appropriate conditioning of the pseudosclerotia (see Favorable Environmental Conditions), stipe development occurs in two well-defined stages: germination and emergence. During germination, dark, hardened stipe initials, also known as fundaments, develop from the overwintering pseudosclerotium (Figure 6). These fundaments grow up to 5 mm in length during the germination phase, and then continue to grow and elongate (between 5-15 mm) in the emergence phase. Once fundaments become fleshy and a depression at the tip is formed, they are referred to as stipes (Figure 7). This depression later becomes an apothecium. Rhizoidal tufts are commonly found at the junction between the base of the stipe and the pseudosclerotium. It has been proposed that these are used for water uptake during apothecium development and ascospore production.
|

Figure 6
|

Figure 7
|
Asexual Reproduction
Conidia are round, hyaline asexual spores that are vectored by pollinators and infect blueberry flowers. Honey divided the genus Monilinia into two groups- the Junctoriae and the Disjunctoriae. M. vaccinii-corymbosi is classified within the Disjunctoriae, which is distinguished by the presence of disjunctors or specialized separating structures between conidia. The disjunctors are thought to aide in dissemination and may detach with the spore when dislodged.
Once a conidium has been successfully vectored to the host’s stigmatic surface, it germinates to form a germ tube. Unlike closely related Monilinia species in the Junctoriae, the germ tube of M. vaccinii-corymbosi grows unidirectionally down the style, much like a pollen tube. By adhering selectively to imprints of stylar transmitting tract tissue, the fungus can gain entry into the ovary without destroying the canal for the later pollen tube growth and fertilization needed for berry formation and subsequent colonization.
Disease Cycle and Epidemiology
The Disease Cycle
The disease may be infrequently observed after planting; therefore, it may not be an economic problem for many years. If growers do not manage the disease, inoculum will slowly build up over time. A year of highly favorable weather will result in dramatic increases in mummy berry and cause economic losses for the grower from that time on.
Pseudosclerotia present on the soil surface germinate in early spring to form apothecia, which can discharge up to 650,000 ascospores (Figure 8). Ascospores can remain viable for between 14 and 48 days under laboratory conditions. Viability is temperature dependent and cooler temperatures (~5°C) were found to be the most conducive to longevity. Floral buds become susceptible as bud scales begin to separate and vegetative buds become susceptible when 2-5 mm of green tissue becomes visible. Ascospores land on this new floral and vegetative tissue, germinate under appropriate environmental conditions, and gain entry to the host either directly through the epidermis or indirectly via stomata. This initiates primary infection.
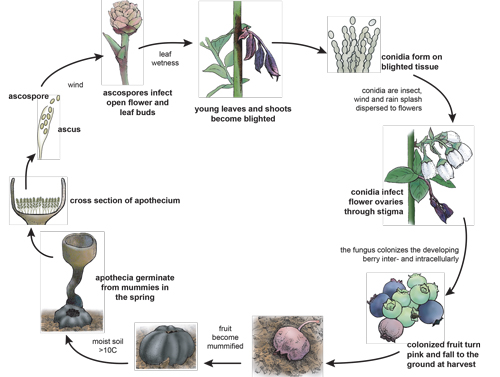
Figure 8
Infected shoots, leaves, and twigs become covered with a grayish mat of conidia, which are then vectored, primarily by pollinators, to healthy blueberry flowers at anthesis. The conidium is deposited on the stigma of the blueberry flower, and it then germinates to form a germ tube. The germ tube grows unidirectionally down the style of the host toward the ovary, adhering to the stylar canal. Hyphal growth rates are correlated with blueberry flower age, with the fastest growth rate associated with the youngest blossoms. Thus, the youngest blossoms are most susceptible to flower infection. Once the flower is fertilized, the fungus will begin to grow, invading the locules and replacing the fruit with a dense mat of hyphae. The blueberry will begin to shrivel as the mat of hyphae expands, and the newly formed pseudosclerotium will drop to the ground around the time of harvest.
From late summer until early spring, the pseudosclerotium will rest on the blueberry orchard floor, detecting environmental changes. During this time, the pseudosclerotium will undergo several transitions such as shedding the outer blueberry skin, increased melanization, germination, and emergence.
In early spring, under conditions of adequate soil moisture and temperature, the pseudosclerotium will form apothecia that will disseminate ascospores and begin the cycle again. A 42% soil moisture content (SMC) measured by weight has been shown to be conducive to stipe production in a laboratory setting. In addition to SMC, a temperature below 21 °C is conducive to stipe production, 10°C being the most productive temperature. These temperatures satisfy the degree-day requirement of Monilinia vaccinii-corymbosi, or days with an average temperature at or above 7.2°C needed for apothecium development.
Aerial Dispersal
Once the apothecium is formed, ascospore release is achieved in conditions of low relative humidity and low wind speed. Ascospores are long-lived (up to 48 days when stored at a temperature of 5°C under laboratory conditions), and are well-suited for wind dispersal and long-distance transport. In field studies, increased blight incidence downwind as compared to upwind of an ascospore point source is further evidence that ascospores are forcibly ejected from asci up and into wind currents, then disseminated. In the same study, there was a tendency for more fruit infections to occur upwind of a conidia point source. The upwind pattern of conidia dissemination is similar to that seen in bee foraging, but results also provide evidence for some wind dissemination.
When conidia of M. vaccinii-corymbosi were examined by Woronin, he observed disjunctors, which function as separators between each conidium on the conidial chain. As the disjunctors elongate, it becomes easier for each conidium to break apart from the chain. At the time, Woronin postulated that this appeared to be an adaptation for wind dissemination. However, the disjunctors could also facilitate spore transportation by insects (see A Case of Floral Mimicry).
Favorable Environmental Conditions
Pseudosclerotia of M. vaccinii-corymbosi overwintering on the blueberry orchard floor are affected by a variety of environmental cues. During late summer, when the pseudosclerotium has dropped from the host blueberry bush, increased soil moisture is associated with higher rates of pseudosclerotial decay for immature pseudosclerotia. Therefore, mature black pseudosclerotia have a higher likelihood of survival.
During the winter, pseudosclerotia are conditioned by chill-hours (mean temperature <7.2°C). The chill-hour requirement in North Carolina is from 900 to 1,200 hours, while the requirement in the state of Washington is 700 hours. Chill-hour satisfaction and high levels of soil moisture enhance carpogenic germination. Degree-day accumulation (days with an average temperature at or above 7.2°C), adequate soil moisture, and exposure to light promote apothecium production. Ascospore dissemination is enhanced in environments with low relative humidity.
In Oregon, apothecia emergence in one blueberry field infected with mummy berry was followed over a 9 year period by researchers at Oregon State University. The intervals of time when apothecia could be found in the field were compared with data on rainfall and temperature during the same interval. The shortest periods of apothecia production occurred in years that were particularly warm and dry (average temp of 11°C and <8 cm of precipitation). The longest periods of apothecia production occurred in years that were wet and cool (average temp of 8°C and >8 cm of precipitation). Infection periods lasted from 8 days during one dry warm year and 4 weeks in another cool wet year.
Environmental information pertaining to disease sporulation has been utilized to create the Mummy Berry Forecast System in Nova Scotia, Canada (Table 1). By visually assessing when the floral bud scales have separated and leaf buds are more than 0.6 cm green, growers can determine when 40-50% bud break has occurred. If temperature and leaf wetness fall within the moderate to high infection range on the table, it is recommended that the grower apply a fungicide within 72 h from the start of the wet period.
| Table 1. Wetting duration (in hours) associated with mummy berry infection at different air temperatures in Nova Scotia, Canada (Data from Paul Hildebrand and Rick Delbridge). |
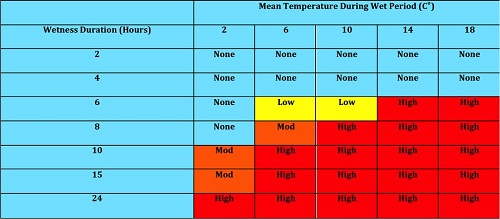 |
Disease Management of Mummy Berry Disease
Cultural Control
Since the conidia of M. vaccinii-corymbosi are pollinator-vectored and, to a lesser extent, wind dispersed, dispersal of secondary inoculum by pollinator vectors is impossible to prevent without harming the vector, thus reducing pollination and impeding adequate fruit set. Therefore, disease management by focusing on the overwintering stage (pseudosclerotia) is key.
Experiments in North Carolina have shown that burial of M. vaccinii-corymbosi under at least 2.5 cm of soil completely inhibited pseudosclerotial germination. This research is often applied to the field, and many farmers utilize post-harvest cultivation. Field research has determined that mechanical cultivation of pseudosclerotia can reduce apothecial emergence by about 50%. Of the three cultivation methods examined, a rotary cultivator was more effective than a disc harrow or in-row cultivator at burying pseudosclerotia, with the percent pseudosclerotia buried below 2.6 cm being 78.6%, 52.6%, and 20.9% respectively. Some farmers also use mulching as a method of burial. Mulching has the added benefit of increasing organic matter content in the soil and water retention around the root zone of the blueberry bush (Figure 9).

Figure 9
Sanitation is another common method of cultural control. Pseudosclerotia can be collected during harvest (or raked into the aisle and gathered); they must be subsequently taken to cull piles, and they must be destroyed or buried before the following spring to prevent newly-formed apothecia from producing ascospores and reinfecting the field. Many farmers have also tried raking or disrupting apothecia in the spring.
Cultivar selection is another important factor in disease management. If a susceptible cultivar of blueberry is heavily infected within the field, the cultivar can be removed to prevent increased disease pressure on less susceptible bushes. If the bushes are to be replaced, planting resistant cultivars such as Reka and Bluejay will reduce losses to mummy berry (Table 2).
| Table 2. Susceptibility of common blueberry cultivars to fruit infection by M. vaccinii-corymbosi. Susceptible, moderately resistant, and resistant varieties mean fruit infection incidence from 1996-1997 was 30-50.9%, 11-29.9%, and 0-10.9% respectively (Data from Stretch and Ehlenfeldt, U.S. Department of Agriculture). |
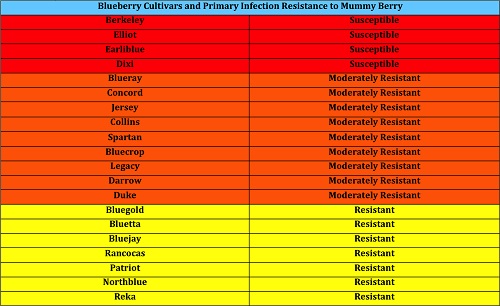 |
Chemical Control
There are several registered fungicides available for control of mummy berry disease. In addition, the use of some desiccants and herbicides may have the added benefit of reducing primary inoculum in the field. Laboratory studies have demonstrated that ammonium thiosulfate (a bloom thinner and desiccant) and diuron (an herbicide), can completely inhibit apothecia maturation when applied to immature stipes. Application can also reduce apothecium longevity when applied directly to open apothecia. Simazine (another herbicide) effects spore viability if applied before germination or at stipe emergence by causing malformed apothecia.
Significance
Losses and Economic Impact
Economic loss due to mummy berry in North America has been documented repeatedly since the late 1960’s. In 1969, British Columbia, Canada experienced an 8.1% crop loss, with an estimated monetary loss of $750,000. In 1974, many commercial farmers in New Hampshire experienced a 70-85% crop loss due to mummy berry. In 1981, mummy berry infection in Nova Scotia caused 10% blight of blueberry bushes in 30% of a 250-hectare field. In 2002, an estimated 70-80% crop reduction was reported on a crop of no-spray rabbiteye blueberry in North Carolina. In Oregon, an 80% crop loss in organic blueberry production is not unusual in a year of disease-conducive environmental conditions.
A Case of Floral Mimicry
Floral mimicry is the close sensory (visual, olfactory, etc.) similarity of one living organism to another, resulting in misidentification by a third organism. In 1985, L.R. Batra ran a group of experiments to test for evidence of floral mimicry during the secondary infection of blueberry by M. vaccinii-corymbosi.
In the first experiment, blueberry shoots were bagged and hand-pollinated to prevent contact with pollinators. When these shoots were compared to shoots that had received insect visitation, 10% infection was observed on bagged trusses as compared to 63% infection on shoots that were left open to pollinators. This showed that mummy berry fruit infection was primarily associated with visitation by pollinators.
Pollinators were observed contacting the conidial mat before flying off to pollinate healthy blueberry flowers. Conidia were subsequently found in the facial hairs of pollinators and an analysis of the conidial mat confirmed the presence of sucrose, glucose, and fructose, all of which are present in the nectaries of Vaccinium. Blighted leaves and shoots also reflected UV in a range similar to that of blueberry flowers. In 1928, Edwin E. Honey commented on the sweet, aromatic odor that wafted from conidial mats that was even present in herbarium samples several years after collection. This scent may also play a key role in pollinator attraction.
Selected References
Ainsworth, G.C., and G.R. Bisby. 1961. A dictionary of the fungi commonwealth. Mycological Institute. Kew, England.
Batra, L.R., and S.W. Batra. 1985. Floral mimicry induced by mummy-berry fungus exploits host’s pollinators as vectors. Science 228:1011–1013.
Batra, L.R. 1983. Monilinia vaccinii-corymbosi (Sclerotiniaceace): Its biology on blueberry and comparison with related species. Mycologia 75:131-152.
Batra, L.R. 1991. World species of Monilinia (Fungi): Their ecology, biosystematics and control. J. Cramer, Berlin.
Bristow, P.R. 1979. Mummy berry disease: Mummy germination. Pages 163-169 in: Proc. North Am. Blueberry Res. Workers Conf., 4th. J. N. Moore, ed. University of Arkansas, Fayetteville.
Cline, W.O. 2003. Harvest Update. North Carolina Cooperative Extension Service. N.C. Blueberry News 8:1-2.
Cox, K.D., and H. Scherm. 2001. Oversummer survival of Monilinia vaccinii-corymbosi in relation to pseudosclerotial maturity and soil surface environment. Plant Dis. 85:723-730.
Drummond, F., J. Smagula, S. Annis, and D. Yarborough. 2009. Organic wild blueberry production. Maine Agricultural and Forest Experiment Station Bulletin 852.
Hildebrand, P.D., and P.G. Braun. 1991. Factors affecting infection of lowbush blueberry by ascospores of Monilinia vaccinii-corymbosi. Can. J. Plant Pathol. 13:232-240.
Honey, E.E. 1928. The Monilioid species of Sclerotinia. Mycologia 20:127-157.
Honey, E.E. 1936. North American species of Monilinia. I. Occurrence, grouping, and life-histories. Amer. J. Bot. 23:100-106.
Kirk, P.M., P.F. Cannon, J.C. David, and J. A. Stalpers. 2001. Ainsworth & Bisby's dictionary of the fungi. 9th ed. Wallingford, UK: CAB International.
Longyear, B.O. 1901. A sclerotium disease of the huckleberry. Annu. Rep. Mich. Acad. Sci. 3:61-62.
Milholland, R.D. 1974. Factors affecting apothecium development of Monilinia vaccinii-corymbosi from mummified highbush blueberry fruit. Phytopathology 64:296-300.
Milholland, R.D. 1977. Sclerotium germination and histopathology of Monilinia vaccinii-corymbosi on highbush blueberry. Phytopathology 67:848-854.
Ngugi, H.K., and H. Scherm. 2006. Mimicry in plant-parasitic fungi. FEMS Microbiol. Lett. 257:171-176.
Ngugi, H.K., and H. Scherm. 2004. Pollen mimicry during infection of blueberry flowers by conidia of Monilinia vaccinii-corymbosi. Physiol. Mol. Plant Pathol. 64:113–123.
Pepin, H.S., and H.N.W Toms. 1969. Economic loss from mummy berry of highbush blue-berry in coastal British Columbia. Canada Plant Dis. Surv. 49:105-107.
Ramsdell, D.C., J.W. Nelson, and R. Myers. 1974. An epidemiological study of mummy berry disease of highbush blueberry. Phytopathology 64:222-228.
Reade, J.M. 1908. Preliminary notes on some species of Sclerotinia. Ann. Mycol. 6:109-115.
Scherm, H., A.T. Savelle, and P.L. Pusey. 2001. Interactions between chill-hours and degree-days affect carpogenic germination in Monilinia vaccinii-corymbosi. Phytopathology 91:77-83.
Shinners, T.C., and A.R. Olson. 1996. The gynoecial infection pathway of Monilinia vaccinii-corymbosi in lowbush blueberry (Vaccinium angustifolium). Can. J. Plant Sci. 76:493-497.
Stretch, A.W., and M.K. Ehlenfeldt. 2000. Resistance to the fruit infection phase of mummy berry disease in highbush blueberry cultivars. HortScience 35:1271–1273.
Wallace, D.B., W.E. MacHardy, and E.M. Meader. 1976. Control of mummy berry of highbush blueberry in New Hampshire. Plant Dis. Rep. 60:97-101.
Wharton, P.S., and A.M.C. Schilder. 2005. Effect of temperature on apothecial longevity and ascospore discharge by apothecia of Monilinia vaccinii-corymbosi. Plant Dis. 89:397-403.
Voronin, M.S. 1888. Über die Sclerotienkrankheit der Vaccinieen-Beeren: Entwickelungsgeschichte der diese krankheit verursachenden sclerotinien. St.-Pétersbourg: Commissionnaires de l'Académie impériale des sciences.
