McCorkle, K.L., Koehler, A.M., Larkin, M., Mendoza-Moran, A., and Shew, H.D. 2019. Petal Blight of Camellia. DOI:10.1094/PHI-I-2019-0702-01
Petal Blight of Camellia
Ciborinia camelliae Kohn (synonyms:
Sclerotinia camelliae Hara and
Sclerotinia camelliae Hansen & Thomas)
Camellia species and hybrids, including
C. japonica,
C. sasanqua, C. hiemalis, C. oleifera, and C.
reticulata.
Authors
Kestrel L. McCorkle1, Alyssa M. Koehler2, Maximo Larkin3, Arlene Mendoza-Moran4, and H. David Shew3
1Greenlight Biosciences, Research Triangle Park, NC, 27709
2Department of Plant and Soil Sciences, University of Delaware, Georgetown, DE 19947
3Department of
Entomology and Plant Pathology, North Carolina State University Raleigh, NCC 27695
4Distance Education and Learning Technology
Applications, North Carolina State University, Raleigh, NC 27695
Introduction

Figure 1: Camellia plant
|
Camellias are popular evergreen shrubs highly prized for their dark green waxy leaves and large colorful flowers (Figure 1).
Camellia species originated in Asia, where they have been used in numerous cultures for centuries. Camellias are popular ornamentals and some species can be used commercially to make tea (C. sinesis L.), while others are used as oils for cosmetics and cooking (C. oleifera Abel). In the landscape, camellias can be accent plants, used to form hedges and borders, or pruned for growth as small trees. Flowers range in size, color, and form. The three most popular ornamental camellia species include
Camellia sasanqua Thunb.,
C. japonica L., and
C. reticulate Lindl. There are additional species and over 20,000 registered hybrids of camellias, most of which are known to be susceptible to petal blight. In the United States, camellias are widely grown in the southeast, and the Pacific and Gulf coasts.
Petal blight is caused by the ascomycete fungus
Ciborinia camelliae (Kohn). The pathogen infects flowers soon after they begin to open (January through April in the northern hemisphere). Infected blossoms turn brown and fall to the ground, and while severe infections can significantly decrease the aesthetic appeal of plants in the landscape, the disease is not harmful to the long-term health of the plant.
Symptoms and Signs
Petal blight symptoms begin within 48 hours of infection as small brownish specks with an uneven margin (Figure 2). In some varieties, veins of infected petals may appear darker than the surrounding tissue giving petals a netted appearance in the early stages of infection (Figure 3). The disease can develop rapidly, completely covering petals with irregular necrotic lesions (Figure 4). Lesions may coalesce, eventually turning the entire flower a dull brown. At first, infected tissue is slippery to the touch, becoming dry or leathery with age. The lack of tissue disintegration in infected areas indicates that the pathogen does not produce large quantities of macerating cell-wall degrading enzymes as it kills the host tissue.

Figure 2: Camellia flower with initial lesion from
Ciborinia camelliae
|

Figure 3: Camellia flower infected with
Ciborinia camelliae
displaying netting in infected petals
| 
Figure 4: Progression of symptoms from individual petal lesions to necrotic flowers in camellia flowers infected with
Ciborinia camelliae
|
Symptoms of petal blight can be confused with cold injury, water spots, or
Botrytis infections. In the case of petal blight, not all blossoms are affected, and the distribution of affected blossoms often appears to be random. Flowers infected by
C. camelliae generally retain their shape and firmness for many days after they have fallen. In contrast, when injured by cold temperatures, most blossoms on the bush or an exposed side of a bush are affected. All symptoms develop at the same time and there are no hyphae at the base of symptomatic flowers (Figure 5). Flowers typically lose their shape and damaged tissue eventually becomes white to light tan in color (Figure 6) . Water spots result in lesions that do not enlarge as those infected with
C. camelliae. Botrytis is another pathogen that can infect camellia petals and cause similar symptoms, but it does not produce sclerotia at the base of the flower like
C. camelliae and typically produces masses of gray sporulation on infected tissues.
Petal blight is most easily diagnosed by looking at the base of symptomatic flowers. After removal of the calyx, a ring of white or gray mycelium can be seen at the base of the petals (Figure 7A-C). A combination of the pathogen mycelium and host tissue form a flattened dark structure called a sclerotium either on individual petals, or more commonly as a structure encompassing the whole base of the flower. The sclerotia are survival structures that darken with age, becoming black and very hard at maturity (Figure 8A-B).
 Figure 5: Camellia flower with frost damage displaying no hyphae at the base of the petals Figure 5: Camellia flower with frost damage displaying no hyphae at the base of the petals
|

Figure 6: Camellia flower displaying light tan discoloration from cold injury
| 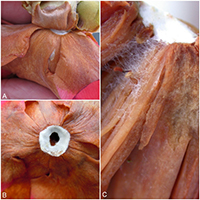
Figure 7: Hyphae of
Ciborinia camelliae on camellia flowers (A) Mycelium at the base of petals (B) Ring of white hyphae at the base of petals (C) Hyphae extending from the base of petals
|
 Figure 8: Sclerotia formation in camellia flowers following infection by
Ciborinia camelliae
(A) Sclerotia forming at the base of a flower (B) Symptomatic flowers and arrows indicating sclerotia of
C. camelliae Figure 8: Sclerotia formation in camellia flowers following infection by
Ciborinia camelliae
(A) Sclerotia forming at the base of a flower (B) Symptomatic flowers and arrows indicating sclerotia of
C. camelliae
|
Pathogen Biology
Ciborinia camelliae is a haploid organism in the kingdom Fungi, phylum Ascomycota, order Helotiales, and family Sclerotiniaceae. This organism is active at 10-24˚C, but optimum temperatures are between 15-18˚C. The pathogen can be grown in culture on many types of media including: potato dextrose agar (PDA), potato sucrose agar (PSA), water agar (WA), malt extract agar (MEA), and oatmeal agar (OA). However, it is most commonly grown on PDA. The pathogen can be stored in potato sucrose broth (195 days), on wheat seeds (168 months), or on PDA (9 months).
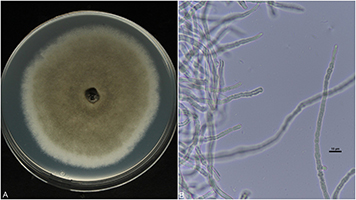
Figure 9: Hyphae of
Ciborinia camelliae
(A) Hyphae growing on potato dextrose agar (PDA) (B) Light microscope image of
C. camelliae
hyphae at 600X total magnification
|
Hyphae
Mycelium found at the base of infected flowers is white to light gray in color with relatively thick hyphal strands. In culture, hyphae begin as white felt-like colonies that darken with age (Figure 9A-B).
Reproduction
The pathogen reproduces sexually through the production of ascospores that are forcibly ejected from apothecia that arise from overwintering sclerotia. Sclerotia are the primary survival structure of the pathogen and can survive in the soil for multiple years. Sclerotia are generally not uniform in size or shape. They are dark in color, typically 12 mm x 10 mm x 2 mm in size, and are formed in the base of infected camellia flowers. A single sclerotium can germinate to form one or up to eight apothecia (Figure 10).
The apothecium is cup-shaped, and ranges in size from 5 to 18mm in diameter. Apothecia are a dull cinnamon color when young and have a cup-shaped appearance (Figure 11). As they age and mature, they become a dull brown and discoid (Figure 12). The apothecial cup is formed on a thin, stalked structure called a stipe. Stipes vary in length depending on how deep the sclerotium is buried in the soil and range from 2 to 100 mm in length and 1 to 2 mm in width.
On the inside of the apothecia cups is the layer where the sexual ascospores are formed. This layer, also known as the hymenium (Figure 13A-B), contains thousands of tiny ascospores that are housed in elongated sacs called asci (Figure 14). In each individual ascus, there are eight ascospores (Figure 15A-B). Ascospores are clear, or hyaline, one-celled, ovate to obovate, have one nucleus, and are 7.5 to 12.5 µm long x 4 to 5 µm wide (Figure 16).
Ciborinia camelliae can produce a microconidial anamorph that is not a part of the infection process. This stage occurs in infected flowers and in some cases in pure culture, and is a part of the sexual reproduction process. Microconidia are catenate, brown-walled, globose to obovate, and 2.5 to 4 µm.
 Figure 10: Germinated sclerotia of Ciborinia camelliae with multiple apothecia Figure 10: Germinated sclerotia of Ciborinia camelliae with multiple apothecia
| 
Figure 11: Young apothecia of Ciborinia camelliae |

Figure 12: Mature apothecia of Ciborinia camelliae
|
 Figure 13: Cross sections of Ciborinia camelliae apothecia (A) Light microscope image of an apothecia cross section at 40X total magnification. Arrows indicating 1. Hymenium 2. Hypothecium 3. Medullary excipulum 4. Ectal excipulum (B) Light microscope image at 100X total magnification stained with Eosin B. Arrows indicating 1. Hymenium 2. Hypothecium Figure 13: Cross sections of Ciborinia camelliae apothecia (A) Light microscope image of an apothecia cross section at 40X total magnification. Arrows indicating 1. Hymenium 2. Hypothecium 3. Medullary excipulum 4. Ectal excipulum (B) Light microscope image at 100X total magnification stained with Eosin B. Arrows indicating 1. Hymenium 2. Hypothecium
|
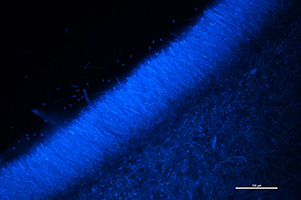
Figure 14: Fluorescent microscope image of multiple asci of
Ciborinia camelliae stained with DAPI at 200X total magnification under a DAPI filter
|
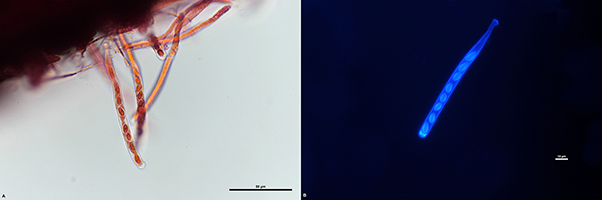
Figure 15: Asci of Ciborinia camelliae (A) Light microscope image of asci showing 8 ascospores per ascus stained with Safranin O at 600X total magnification (B) Fluorescent microscope image of a single ascus with 8 ascospores stained with Calcofluor-white and magnified at 60X under a DAPI filter
| 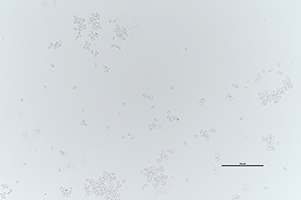
Figure 16: Light microscope image of Ciborinia camelliae ascospores at 200X total magnification |
Nomenclature
The pathogen was originally in the genus
Sclerotinia, C. camelliae was moved into the genus
Ciborinia by Wetzel (1945). The genus
Ciborinia is 1 of 14 genera in the family Sclerotiniaceae, and it contains species that incorporate whole or partially digested host tissue into their sclerotia. There are 20 different species in the genus
Ciborinia and all are host-specific pathogens.
Host Range
Many species of
Ciborinia only have one host, while others may be able to infect a few species in a single genus.
Ciborinia camelliae is a specialist pathogen that only infects the flowers of camellia species. Susceptible species include:
C. japonica, C. sasanqua, C. hiemalis, C. oleifera, and
C. reticulata. Many more camellia species and hybrids are also susceptible to
Ciborinia camelliae and host resistance has not been found. Blight tends to be the most severe in
C. sansanqua, but infection is avoided if flowering does not coincide with spore release.
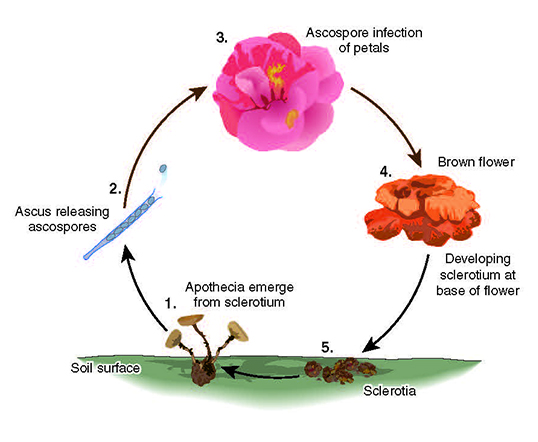
Disease Cycle and Epidemiology
Camellia petal blight is a monocyclic disease that starts with the germination of overwintering survival structures called sclerotia that have been resting in the soil or mulch layer below camellia bushes from previous years.
|

Figure 17: Apothecia of Ciborinia camelliae releasing ascospores
|
As is characteristic of this genus, sclerotia of
C. camelliae are formed from pathogen and host tissue and vary in size and shape (Figure 8B). When camellias start to bloom, if there is high relative humidity, and temperatures are between 15-21˚C, apothecia are formed from germinating sclerotia in the soil (Figure 10). Apothecia are cup-shaped structures that contain ascospores, which are ejected into the air (Figure 17) and float until they land on susceptible host tissue. Over a period of several days, millions of spores are ejected and can travel a few meters up to kilometers (Video 1).
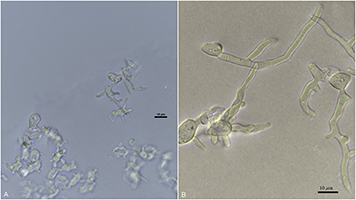
Figure 18: Light microscope image of germinating ascospores of
Ciborinia camelliae (A) 60X magnification of germ tubes after 24 h of germination (B) 60X magnification of extended germ tubes 48 h after germination
|
Ascospores are able to infect any time after tips of camellia petals emerge from their buds and show color. Stamens and sepals also can be infected by
C. camelliae. Once ascospores come into contact with a camellia flower, they germinate to form one or many germ tubes (Figure 18A-B). Six hours after germination, short germ tubes will form and directly penetrate the host cuticle. The hypha will grow under the cuticle towards the junction of the epidermal cells, and after reaching the epidermal cell, the primary hypha branches and grows intercellularly for the next 48-72 hours. During this period, cell walls of the host start to turn brown and after 72 hours a lesion can be observed. Three days post germination, intracellular hyphae can be observed, but the pathogen does not enter epidermal cells.

Figure 19: Camellia flowers infected with
Ciborinia camelliae that have fallen to the ground
|
If colonization of the host is successful, then hyphae combine with host tissue near the base of the flower to form a new sclerotium. New sclerotia form 15-30 days after flowers are infected and fall to the ground where they remain dormant until the next year when the disease cycle starts over again (Figure 19A-B). Most sclerotia produce apothecia the following spring after they are formed and can produce apothecia for several years in a row. Unless sclerotia are removed and destroyed, some can remain viable in the soil or leaf litter for at least 5 years.
Video 1: Apothecia of
Ciborinia camelliae releasing ascospores
Management
Once
Ciborinia camelliae is established in an area, it is unlikely to be eradicated. Therefore, the best way to protect plants in a nursery, or home garden, is to prevent the introduction of the pathogen. The pathogen can be avoided by planting autumn flowering camellia species or varieties that flower very early in the season. In addition to avoidance, there are several cultural and sanitation practices that can reduce disease severity and some available chemical controls.
Cultural and Sanitation Practices
Cultural and sanitation practices are the most effective for controlling camellia petal blight. To avoid introduction of
C. camellia into an area, do not plant infected plants. A good solution is to import and plant camellias that are bare root to avoid bringing in soil that may contain inoculum. However, quarantines that only regulate the movement of the host are often ineffective because of sclerotia movement by people through soil debris in equipment or shoes. Once camellias become infected, flower removal can be an effective sanitation practice to reduce inoculum. Under camellias already present, the removal of weeds and ground covers can aid in finding and removing diseased flowers. Once blooming is over and all flowers have been collected, the top layer of mulch can be raked from under plants and replaced with a 1-2 inch thick layer of new mulch to interfere with spore release the following season. Lower branches of each plant can be pruned to increase air flow and create less obstacles for picking up dead flowers from under the plant. Diseased flowers that are collected should be destroyed and should not be composted because sclerotia can survive the composting process.
Chemical Control
The use of fungicides to control this disease is uncommon and should only be used if severe levels of infection are expected based on high disease levels in previous seasons or for high value plantings. Applications can be made as a ground spray or sprayed directly on flowers. Ground applications should be made in the fall or early winter before flowering by drenching the soil around plants. For control, a 10-foot circle of fungicide must be applied to the soil surface around each plant (usually 2 or more quarts). Ground applications prevent apothecia development, so spores are never released to infect blossoms. Preventative applications can also be made directly to open flowers. Applications should be initiated when buds begin to show color and repeated based on the labeled fungicide interval. Captan and mancozeb can be used, but are usually not cost effective for use in home gardens.
Host Resistance
To date no high levels of resistance has been identified, but different degrees of susceptibility have been observed. Common species
C. sasanqua and
C. japonica are rated as very susceptible. Less susceptible species may have delayed symptoms and/or no formation of sclerotia. Hybrids derived from
C. lutchiensis have been shown to be the most resistant to infection by C. camelliae.
Biological Control
Many organisms have been isolated from decaying sclerotia and uninfected camellia flowers and evaluated for efficacy as biological control agents for
C. camelliae. Organisms evaluated for degrading sclerotia include:
Trichoderma spp.,
Coniothyrium minitans, Clonostachys rosea, Schizophyllum commune Sc3,
Phanerochaete cordylines HR469, and
Pycnoporus coccineus HR582. All organisms tested were effective at reducing the number of viable sclerotia
in vitro, however when evaluated under field conditions, they were not effective at reducing the sclerotia population. Tree mulches produced from pine, gum, kanuka, and a mix of numerous tree species were also tested for efficacy of reducing sclerotial numbers and inhibiting the formation of apothecia. Adding a thick layer of mulch under camellias during dormancy can reduce the incidence of camellia petal blight. When mulches at least 100 mm thick were applied under camellia bushes, apothecial production was completely suppressed. The number of viable sclerotia in the soil also decreased with the addition of mulch. From camellia flowers, several
Pseudomonas and
Bacillus species have been isolated and tested for their ability to prevent infections on camellia flowers. In camellia petal assays, these organisms provided total protection from infection. However, when these microorganisms were suspended in water and applied to plants, they were not able to colonize petals and did not provide protection against camellia petal blight.
Significance
Ciborinia camelliae was first described from Japan in 1919, but it was not widespread there until the 1970s. In the United States, it was first reported in California nurseries in 1938 and soon afterward in Georgia (1948). Following its introduction into the United States, it spread to most areas where camellias are grown within the next 50 years. The pathogen has also been reported in New Zealand (1993) and Europe (1999). In Europe, the pathogen has spread to England, Portugal, Spain, France, Italy, Switzerland, and Germany and is still considered a quarantined organism in many countries.
Selected References:
CABI, 2013. Fallopia japonica. In: Invasive Species Compendium. Wallingford, UK: CAB International. www.cabi.org/isc. http://www.cabi.org/isc/datasheet/49113
Couselo J.L., Vela P., Salinera C., and Mansilla P. 2014. Susceptibility trials of different Camellia species to
Ciborinia camelliae. International Camellia Journal 46:64-68.
Denton-Giles, M., Bradshaw, R. E., and Dijkwel, P. P. 2013.
Ciborinia camelliae (Sclerotiniaceae) induces variable plant resistance responses in selected species of Camellia. Phytopathology 103:725-732.
Jeffers, S. N., Baxter, L. W. 2001. Diseases of woody ornamentals and trees in nurseries. Chapter 27, 100-101. The American Phytopathological Society Press, St. Paul, MN.
Sinclair, W. A., Lyon, H. H. 2005. Diseases of trees and shrubs. Cornell University Press. Second Edition p. 70.
Taylor, C. H., Long, P. G. 2000. Review of literature on camellia flower blight caused by
Ciborinia camelliae. New Zealand Journal of Crop and Horticultural Science 28:2, 123-138.
Van Toor, R. F., Dench, M. W., Jaspers, M. V. and Stewart, A. 2005. Tree mulches reduce sclerotial numbers and apothecial production by
Ciborinia camelliae. New Zealand Journal of Crop and Horticultural Science 33:2, 161-168.
Van Toor, R. F., Jaspers, M. V., and Stewart, A. 2005. Effect of soil microorganisms on viability of sclerotia of
Ciborinia camelliae, the causal agent of camellia flower blight. New Zealand Journal of Crop and Horticultural Science 33:2, 149-160.
Van Toor, R. F., Jaspers, M. V., and Stewart, A. 2005. Wood rotting fungi and pine mulches enhance parasitism of
Ciborinia camelliae sclerotia
in vitro. New Zealand Journal of Crop and Horticultural Science 33:4, 389-397.
Van Toor, R. F., Pay, J. M., Jaspers, M. V., and Stewart, A. 2005. Evaluation of phylloplane microorganisms for biological control of camellia flower blight. Australian Plant Pathology Society 34:525-531. Vingnana-Singam, V., Long, P. G., and Rowland, R. E. 2000. Infection processes of
Ciborinia camelliae on camellia flower tissue. New Zealand Plant Protection 53:151-156.
