Pine wilt disease
Bursaphelenchus xylophilus
Scots, Austrian, jack, mugo, and red pines and, less commonly, white pines.

Pine wilt disease. (Courtesy P. Donald, copyright-free)
Symptoms and signs
Pine wilt is a dramatic disease that typically kills affected trees within a few weeks to a few months (Figure 1). The causal pathogen is the pine wood nematode (PWN), Bursaphelenchus xylophilus. Most plant-parasitic nematodes are associated with plant roots, but the pine wood nematode is found in aboveground parts of the tree. Nematodes kill the tree by feeding on the cells surrounding the resin ducts. This causes resin to leak into the tracheids, resulting in "tracheid cavitation" or air pockets in the water transport system. Just as a person cannot drink through a straw with holes in it, the tree cannot move water upward and consequently wilts and dies. Bursaphelenchus xylophilus is transmitted by vector pine sawyer beetles in the genus Monochamus. The dispersal stage of the nematode is carried in the insect’s respiratory system and thus spread from tree to tree as the beetles feed on the young shoots of pine trees.

Figure 1 |
Tree death usually progresses from the top of the tree downward, distinguishing this disease from needle diseases. Needle discoloration is usually the first symptom. Needles change from their normal color to a grayish green color and finally a tan to brown color. Retention of dead needles on the branch is another diagnostic feature. In some cases, no disease progression will be seen following the death of an individual branch until the next growing season.
The pine wood nematode is widely distributed in the United States, but the highest incidence of the disease is in the Midwest. The disease is most serious on pine species that are not native to North America, with the greatest impact in landscape plantings and windbreaks. Age of the tree also influences susceptibility, with an increased risk of developing pine wilt when trees are greater than 10 years of age. Worldwide, the problem is epidemic in Japan and other parts of Asia, where it is the native pine forests that are at risk. Scots pine is considered the most susceptible species in the United States, although many of the other species in the Pinus (Sylvestres) subsection (e.g., Austrian, mugo, and Japanese black pines) are also highly susceptible. In contrast, species in the Australes subsection (e.g., loblolly, longleaf, pitch, and slash pines) are generally highly resistant.
Diagnosis requires observation and identification of the nematode, which can be recovered from cross sections of symptomatic limbs greater than 2.5 cm (1 in.) in diameter soaked in water. The greatest success in finding the nematode involves soaking a trunk section in water for 24 to 48 h. One can also sample wood by drilling into branches or the trunk with a large diameter (1-2.5 cm/ 0.5-1 in.) auger drill bit and soaking the resulting wood chips in water. Nematodes may not be well distributed throughout the tree, so it may be necessary to test several samples from different areas of the tree to find the nematodes. Microscopic examination of the liquid is needed to identify the nematode.
Several different bacterial- and fungal-feeding nematode species can inhabit the wood of dead or dying pine trees. Removal of bark before the samples are soaked can reduce the number of these non-target nematodes. There can also be several species of Bursaphelenchus present, but only B. xylophilus is recognized as the causal agent of pine wilt.
Morphology of B. xylophilus
The pine wood nematode is a microscopic unsegmented worm about 1 mm in length. During early stages of the disease, nematode populations recovered from wood samples consist of adult males (Figure 2) and females (Figure 3), along with propagative second-stage (J2) through fourth-stage (J4) juveniles. Identification is based on combined diagnostic traits, including male spicule shape (Figure 2) and female tail shape. Considerable taxonomic expertise is necessary to distinguish among the ten morphologically similar species in the xylophilus group. Morphological variation within B. xylophilus itself further complicates diagnosis. Mucronate (M-form) and round tail (R-form) isolates occur in North America and Japan, for example, with the M-form generally considered to be less pathogenic.
As the disease progresses and population increase within the tree leads to overcrowding, propagative J2 develop into dispersal J3 that exhibit a thickened cuticle and resistance to dessication. Dispersal stages are easily distinguished by the deposition of lipid droplets in the intestine. Nematode populations from samples collected in November or later may consist almost exclusively of the J3 dispersal stage. The dispersal J3 aggregate around the pupal chambers of the insect vector and are induced by stimuli released during beetle eclosion to molt to the dispersal J4 stage. The dispersal J4 stage, which can be recovered from pupal chambers or adult beetles, lacks a stylet and has a degenerate esophagus (Figure 4).
Pathogen Biology
Pine wilt etiology encompasses interactions among the pine wood nematode (Bursaphelenchus xylophilus) (Figures 2 and 3), pine sawyer beetles (Monochamus spp.) (Figure 5), a susceptible coniferous tree, and frequently one or more blue-stain ophiostomatoid fungi (e.g., Ceratocystis and Ophiostoma spp.) (Figure 6). The nematode feeds on living plant cells surrounding the resin canals of pines until tree death, at which point, fungi that have proliferated in the dying tree provide a secondary food source. The interaction between the nematode and beetle has been described as mutualistic, whereby the nematode is transported from host to host by the insect vector, and nematode damage to susceptible pine trees creates new sites for vector oviposition and proliferation.
Bursaphelenchus xylophilus is one of 75 described species of Bursaphelenchus, most of which have a phoretic relationship with insects but are not known to cause disease in trees. It was first described in 1934 (as Aphelenchoides xylophilus) based on specimens recovered from blue-stained longleaf and shortleaf pine logs collected across the southeastern United States, but was only later identified (as B. lignicolus) as the cause of pine wilt - first in Japan in 1971, and then in Missouri in 1979. Results of studies of the genetic diversity of PWN populations suggest that the nematode was introduced into Japan from the United States, where it subsequently spread to China and Korea, and, more recently, to Portugal and Spain.
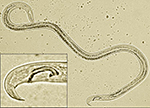
Figure 2 |
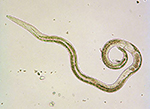
Figure 3 |
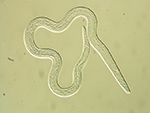
Figure 4 |

Figure 5 |

Figure 6 |
Vector relationships
The dispersal J4 of B. xylophilus are carried in the tracheae of the beetle's respiratory system (Figure 7). When the beetles feed on branches of healthy trees, the nematodes emerge and enter the trees through feeding wounds created by the beetles. The adult sawyers are attracted to recently dead or dying trees for oviposition (egg laying). Trees with bark are necessary for oviposition and larval development. The beetle larvae feed several weeks in the cambial wood and then bore into the sapwood.
At least six North American species of Monochamus have been identified as pinewood nematode vectors, with M. carolinensis and M. scutellatus representing the principal vectors in the United States. An additional six species have been identified as vectors in Asia and Europe, with M. alternatus and M. galloprovincialis being the most important vectors in Asia and Europe, respectively. Vector effectiveness is likely related to a combination of factors, including host preference, beetle generation time, and nematode carrying capacity. Many Monochamus species are polyphagous, however, and the primary vector species encompass a range of generation times, as well as both facultative and obligate diapause. The major vector species do appear to be capable of carrying much larger nematode loads (15-20 thousand per insect) compared to less important vector species.
Spread of the nematode from tree to tree occurs via the pine sawyer beetle (Monochamus spp.). The nematodes are carried in the tracheae of the beetle's respiratory system (Figure 7). When the beetles feed on branches of healthy trees, the nematodes emerge and enter the trees through feeding wounds created by the beetles. The adult sawyers are attracted to recently dead or dying trees for oviposition (egg laying). Trees with bark are necessary for oviposition and larval development. The beetle larvae feed several weeks in the cambial wood and then bore into the sapwood.
At least six North American species of Monochamus have been identified as pinewood nematode vectors, with M. carolinensis and M. scutellatus representing the principal vectors in the United States. An additional six species have been identified as vectors in Asia and Europe, with M. alternatus and M. galloprovincialis being the most important vectors in Asia and Europe, respectively. Vector effectiveness is likely related to a combination of factors, including host preference, beetle generation time, and nematode carrying capacity. Many Monochamus species are polyphagous, however, and the primary vector species encompass a range of generation times, as well as both facultative and obligate diapause. The major vector species do appear to be capable of carrying much larger nematode loads (15-20 thousand per insect) compared to less important vector species.
Population dynamics
Once introduced into a tree seedling, the nematodes migrate mainly via the cortical resin canals in branches. Cortical tissue is not present in stem tissues of trees over four years of age, and therefore it is not likely that the nematodes spread within mature trees via cortical tissue. It has been suggested that spread within mature trees occurs through xylem resin canals. Recent observations confirm that dispersing nematodes first invade cortical resin canals and surrounding cortical tissues, and subsequently spread from cortical resin canals to xylem resin canals and surrounding vascular tissues. When not feeding on the plant cells, the nematodes feed on fungi present within the wood. Bark beetles carry blue-stain fungi to trees, and these fungi, along with other wood-inhabiting fungi, colonize the tree after it is weakened or killed. Research indicates that there is an increase in nematode reproduction when a blue-stain fungus is the food source for the nematode.
The plant-feeding phase of the nematode, B. xylophilus, is usually characterized by rapid reproduction of the nematode and tree death. Laboratory studies show that reproduction on fungi at 25°C (77°F) can progress from egg to adult in four to five days; however, generation time is temperature-dependent. Measurements on pine resin canal tissue are much harder and thus direct comparisons cannot be made here. The nematode goes through four juvenile stages punctuated by molts before becoming an adult. This pathway for the nematode life cycle can continue as long as conditions are favorable for growth and reproduction.
Tree death or scarce food supply results in the nematode switching to a dispersal mode, which is characterized by a close physiological relationship between the nematode and the insect vector. The nematode will only molt into fourth-stage dispersal juveniles in the presence of pine sawyer beetles, and the insect only vectors this J4 stage. The dispersal juveniles aggregate around the pupal chambers of the beetles, and migrate toward the insect tracheae located in the spiracles. The adult beetles chew their way through the sapwood and exit the tree. Carrying the nematodes, adult beetles fly to healthy trees to feed or to dead or dying trees to oviposit.
Pathogenicity
Symptom development is associated with the destruction of cortical and cambial tissues, followed by cavitation-induced embolism of xylem tracheids. Phytotoxins have long been implicated due to the rapid nature of tree death, but gene expression profiles of resistant and susceptible Japanese black pine trees support the hypothesis that a hypersensitive response-like reaction is responsible. As the nematode spreads throughout a susceptible tree, it induces a cascading series of hypersensitive reactions in xylem parenchyma cells resulting in leakage of cell contents (including defense compounds) into tracheids, followed by disruption of water conduction and, ultimately, tree death.
Isolates of PWN vary in their host specificity and virulence. Isolates from balsam fir and Scots pine, for example, are pathogenic on their original host but not on the other conifer species. Virulent and avirulent pathotypes have been recovered from various pine species in both the United States and Japan. Avirulent pathotypes have lower transmission rates, as well as lower rates of migration and reproduction within the tree, resulting in greatly reduced capacity to induce wilting.

Figure 7 |
Disease Cycle and Epidemiology
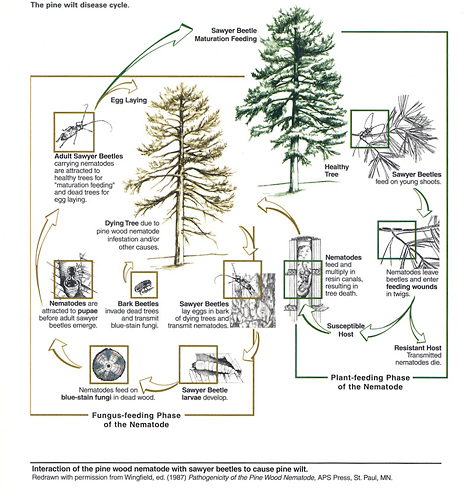
Figure 8
Several months of hot, dry weather are necessary for pine wilt development and spread. Nematode dispersion and multiplication are key factors in disease symptoms and tree death. The pine wood nematode can be a primary or secondary invader of plant tissue (Figure 8); its presence in dead or dying wood, therefore, is not proof that the nematode killed the tree.
Adult beetles feed on bark of young branches (Figure 9) and the nematodes present in the insect spiracles (Figure 7) enter the tree through feeding wounds caused by the beetle. Inside a susceptible tree, the nematodes develop into adults and migrate throughout the tree, feeding on parenchyma cells of the ray canals and reproducing. When conditions favor the nematode (susceptible host, optimal temperature, virulent nematode isolate), internal host responses can be seen within one to three days after infection. These include cell death, increased host respiration, decreased water conductivity, increased ethylene production, and the accumulation of phytotoxic compounds. Trees infected in this manner usually wilt and die rapidly. The nematode is the primary pathogen in this case, and the result is pine wilt. As stated previously, it has been theorized that in susceptible trees the rapid spread of the nematode throughout the tree triggers widespread hypersensitive reactions in cells exposed to the nematode. The combination of nematode migration and a strong defense response essentially results in self-inflicted host mortality.
Infected trees do not all die at the same rate. It is not uncommon for pine wilt disease progression through an entire windbreak, for example, to require a decade or longer. Some infected trees serve as latent carriers, surviving for a year or more without displaying visible symptoms.
The pine wood nematode can also be a secondary invader of dead or dying trees when the dispersal J4 stage enters the wood during sawyer beetle oviposition (Figure 10). In these cases, the cause of tree death may or may not have been pine wilt disease. The resulting populations, however, still serve as potential reservoirs for later dispersal to healthy trees by sawyer beetles.

Figure 7 |

Figure 9 |

Figure 10 |
Disease Management
A successful management strategy targets both the beetle vector and the nematode pathogen. Timely removal and destruction of trees killed by pine wilt disease eliminates the breeding habitat of the vector and prevents nematode transmission to healthy trees. Homeowners should be discouraged from planting susceptible tree species as ornamentals, but valued existing trees can be protected with preventative nematicide injections.
Genetic resistance
Tree breeders in Japan have developed resistant varieties of P. thunbergii (Japanese black pine) from surviving forest trees in pine wilt epidemic areas, but the utility of this approach remains limited and uncertain. Meanwhile, there are many tree species that homeowners can plant to reduce or eliminate the risk of pine wilt disease. Eastern white pine, jack pine, loblolly pine, lodgepole pine, pitch pine, and ponderosa pine are generally considered to be moderately to highly resistant, although conflicting reports of susceptibility exist in some cases. Additionally, while B. xylophilus has occasionally been isolated from conifers other than pines, including larch, fir, and spruce, pine wilt disease has not been reported for any of these species. Norway and blue spruce, balsam fir, and Douglas fir are commonly recommended as replacement trees for Scots and Austrian pines in the Midwestern United States.
Cultural practices
Pine wilt management in the United States is currently best achieved by removal of symptomatic trees. Adult beetles are attracted to recently dead, dying, or freshly cut wood (Figure 11), so dead trees should be cut down and the wood burned or buried as soon as possible. It is important that the stumps also be removed or buried, as these are attractive to the sawyer beetles. The beetles are active from spring through fall and, depending on temperature, can produce several generations during a growing season. Spread of the disease from infected trees to healthy trees can therefore occur anytime until the onset of winter.

Figure 11 |
In areas of the world where susceptible trees are common and the disease is not present (e.g., northern Europe and Scandinavia), avoidance is the primary means of control. There are strict quarantines on the import of wood into these countries. Canadian and U.S. exporters continue to work with researchers to develop wood treatments that kill the nematodes present in wood chips or other unseasoned lumber (Figure 12). Currently, heat-treating unseasoned lumber to a core temperature of 56°C (133°F) for 30 minutes is sufficient to satisfy the European Union requirement that wood products are free of living nematodes or their beetle vectors. Alternative treatments, such as the use of microwaves for heat treatment of wood, are also being investigated. Phytosanitary inspections of forests, lumber, and wood packing materials have been established to prevent the movement of B. xylophilus between countries.

Figure 12 |
Chemical control
In the United States, nematicide and insecticide treatment of trees historically has been considered economically unfeasible, if not ineffective. More recently, however, demonstration of effective PWN control using preventative injections of abamectin, a natural fermentation product of the soil bacterium Streptomyces avermitilis with nematicidal properties, has resulted in several commercial products and injection systems. Survival rates of PWN-inoculated Scots pine trees, as well as mature Scots pine trees exposed to a natural epidemic, have been observed to triple following abamectin injections. Curative injections after symptoms have appeared, in contrast, are ineffective.
In Japan, where the disease is much more devastating, insecticide and nematicide treatments, biological control, and induced resistance through inoculation with less virulent strains of Bursaphelenchus have been investigated.
Significance
In the Midwestern United States, the disease has the greatest impact on homeowners and in planted natural areas due to the prevalence of susceptible, non-native, and poorly adapted pine species. Use of Scots pine in windbreaks and landscape plantings has been especially common in the Midwest, where this tree species was considered to be well adapted to the environmental conditions. Stands of single tree species (monocultures), in particular, provide an excellent breeding site for the insects and associated nematodes. Because Scots pines become more susceptible after they reach 10 years of age, large, well-established trees are disproportionately affected (Figure 1) and their removal has considerable impact on the landscape.
Scots pine is also the tree of choice for Christmas tree plantations in the Midwest. Since most Christmas trees are harvested before they reach 10 years of age, pine wilt epidemics are relatively rare. Abandoned Christmas tree plantations (Figure 13), however, along with homeowner properties and public areas where dead trees are left standing, continue to represent serious threats to existing Scots pines.
The disease has a different impact on U.S. exporters of wood products than on homeowners. Bursaphelenchus xylophilus is listed as a quarantine pest by more than 40 countries. Because of the number of susceptible pine hosts and suitable insect vectors in Europe, the European Union in 1993 imposed a ban on the import of untreated and unseasoned wood from areas where the pine wood nematode was known to occur. Softwood exports from the U.S. and Canada declined by approximately one-third and two-thirds, respectively, the following year.
The impact of pine wilt is greatest outside of the U.S. and Canada. The disease has had a devastating impact on native forests in Japan, China, Korea, and Taiwan. In Japan, where 28% of the total pine forest area is estimated to be infected with B. xylophilus, tens of millions of dollars are spent annually to control the nematode. Additional tens of millions of Euros have been spent on control measures following the establishment of B. xylophilus in Portugal and Spain.

Figure 13 |
Selected References
Akbulut, S., and Stamps, W. T. 2012. Insect vectors of the pinewood nematode: a review of the biology and ecology of Monochamus species. Forest Pathology. 42:89-99.
Bolla, R., Winter, R. E. K., Fitzsimmons, K., and Linit, M. J. 1986. Pathotypes of pinewood nematode Bursaphelenchus xylophilus. Journal of Nematology. 18:230-238.
Braasch, H. 2008. The enlargement of the xylophilus group in the genus Bursaphelenchus. Pages 139-149 in: Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems. M. M. Mota and P. Vieira (eds.). Springer Science & Business Media, Berlin.
Dropkin, V. H., and Foudin, A. S. 1979. Report of the occurrence of Bursaphelenchus lingnicolus induced pine wilt disease in Missouri. Plant Dis. Rept. 63:904-905.
Dwinell, L. D. 1997. The pinewood nematode: regulation and migration. Annu. Rev. Phytopathol. 35:153-166.
Futai, K. 2013. Pine wood nematode, Bursaphelenchus xylophilus. Annual Review of Phytopathology. 51:61-83.
Gleason, M., Linit, M., Zriba, N., Donald, P., Tisserat, N., and Giesler, L. 2000. Pine wilt: A fatal disease of exotic pines in the Midwest. Sustainable Urban Landscapes 9. Iowa State University, Ames.
Hirao, T., Fukatsu, E., and Watanabe, A. 2012. Characterization of resistance to pine wood nematode infection in Pinus thunbergii using suppression subtractive hybridization. BMC Plant Biol. 12:13–27.
James, R. F., Tisserat, N. A., and Todd, T. C. 2006. Prevention of pine wilt of Scots pine (Pinus sylvestris) with systematic abamectin injections. Arboriculture & Urban Forestry. 32:195-201.
Kusumoto, D., Yonemichi, T., Inoue, H., Hirao, T., Watanabe, A., and Yamada, T. 2014. Comparison of histological responses and tissue damage expansion between resistant and susceptible Pinus thunbergii infected with pine wood nematode Bursaphelenchus xylophilus. J. For. Res. 19:285–294.
Linit, M. J. 1988. Nematode-vector relationships in the pine wilt disease system. J. Nematol. 20:227-235.
Mamiya, Y. 1985. Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus. Annu. Rev. of Phytopathol. 21:201-220.
Myers, R. F. 1985. Pathogenesis in pine wilt caused by pinewood nematode Bursaphelenchus xylophilus. J. Nematol. 20:236-244.
Son, J. A., Komatsu, M., Matsushita, N., and Hogetsu, T. 2010. Migration of pine wood nematodes in the tissues of Pinus thunbergii. J. For. Res. 15:186–193.
Steiner, G. and E.M. Buhrer. 1934. Aphelenchoides xylophilus n. sp., a nematode associated with bluestain and other fungi in timber. J. Agric. Res. 48:949-951.
Toth, A. 2011. Bursaphelenchus xylophilus, the pinewood nematode: its significance and a historical review. Acta Biol. Szeged. 55:213-217.
