Potato spindle tuber
Potato spindle tuber viroid
potato, tomato, ornamentals (Solanaceae)
Symptoms and Signs
The natural host range of PSTVd includes many solanaceous species. The viroid may cause disease in
Solanum tuberosum (potato),
S. lycopersicum (syn. Lycopersicon esculentum, tomato), and
Capsicum annuum (pepper) where symptoms may vary considerably depending on plant species, variety, viroid strain and environmental conditions. Infections in other hosts are symptomless; e.g.,
Brugmansia spp.,
Datura sp.,
Lycianthes rantonneti (syn.
S. rantonneti),
Persea americana (avocado),
Physalis peruviana (Cape gooseberry),
S. jasminoides,
S. muricatum (pepino), and
Streptosolen jamesonii.
In potato, growth of infected plants may be severely reduced or even cease entirely; however, reduction in growth may also be hardly visible. As shown in (Figure 1), the vines of infected plants may be smaller, more upright, and produce smaller leaves than their healthy counterparts. Infected tubers may be small, elongated (from which the disease derives its name), misshapen, and cracked. Their eyes may be more pronounced than normal and may be borne on knob-like protuberances that may even develop into small tubers.

Figure 1A |
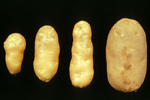
Figure 1B |

Figure 2 |
The first symptoms of PSTVd infection in tomato (Figure 2) are growth reduction and chlorosis in the top of the plant. Subsequently, this growth reduction may develop into stunting, and the chlorosis may become more severe, turning into reddening and/or purpling. In this stage, leaves may become brittle. Generally, this stunting is permanent; occasionally, however, plants may either die or partially recover. As stunting begins, flower and fruit initiation stop.
Peppers display only very mild symptoms in response to PSTVd infection. The only visible symptom is a certain "waviness" or distortion of the leaf margins near the top of infected plants. Infection of many solanaceous ornamentals is often symptomless (Figure 3).

Figure 3B |

Figure 3B |
Pathogen Biology
Unlike the genomes of conventional RNA viruses, the small circular RNA genome of PSTVd encodes no proteins, and no virus-like particles can be isolated from infected tissue. PSTVd RNA consists of 341-364 nucleotides, whereas
Tobacco mosaic virus contains 6,400 nucleotides, and the range for viruses is from 1,400 to 20,000 nucleotides. Visualization in the electron microscope reveals a highly-structured, rod-like native conformation that is 37±6 nm in length; partial denaturation of PSTVd yields a series of intermediate structures containing one or more short hairpin stems (Figure 4).
Comparative sequence analysis suggests that PSTVd and other pospiviroids contain five structural domains. From left to right, these are known as the terminal left, pathogenicity, central, variable, and terminal right domains. Site-directed mutagenesis has demonstrated that certain motifs within one or more of these domains play essential roles in replication, cell-to-cell movement, and disease induction. Sequence changes within a so-called "loop E motif" located in the central domain of PSTVd lead to dramatic changes in both host range and symptom expression. Changes elsewhere in the molecule can alter the ability of PSTVd to enter/exit the vascular system. Like most conventional RNA or DNA plant viruses, PSTVd moves long distances in the phloem.
PSTVd replication occurs in the nuclei of infected cells and is catalyzed by RNA polymerase II, a host-encoded DNA-dependent polymerase normally involved in mRNA synthesis. Replication initiates within the left terminal loop and proceeds via an asymmetric rolling circle mechanism in which only the genomic RNA is present in a circular form (Figure 4). Unlike members of the second family of viroids (i.e., the
Avsunviroidae) that replicate in the chloroplast and contain ribozymes, cleavage of the resulting multimeric progeny RNA requires one or more host-encoded enzymes. Evidence has recently been presented for the involvement of a type-III RNase (i.e., a DICER-like enzyme) in this cleavage reaction. PSTVd replication is accompanied by the synthesis of small PSTVd-related RNAs derived from both the genomic and anti-genomic strand. RNA silencing appears to play a major role in disease induction, because transgenic plants that express small PSTVd-related RNAs in the absence of active viroid replication exhibit symptoms very similar to those seen in infected plants.
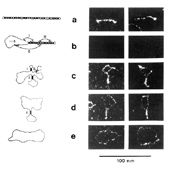
Figure 4 |
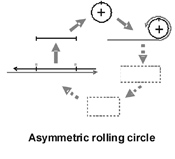
Figure 5 |
Disease Cycle and Epidemiology
Transmission
PSTVd can be transmitted in four different ways:
-
Vegetative propagation. Propagation by tubers, cuttings, and micro-plants provides a very efficient means of viroid transmission. Once established, PSTVd infection is persistent; therefore, plants from infected lots act as a permanent source of inoculum for other lots and crops. Vegetative propagation has been the major pathway for PSTVd transmission in potato and ornamentals such as
Brugmansia spp. and
S. jasminoides. The absence of symptoms increases the risk that infected plants will be used for propagation.
-
Mechanical transmission. Under favorable conditions, PSTVd is readily transmitted by normal cultivation activities. This is most clearly seen with potatoes and tomatoes, where viroid spread is mainly along the row (Figure 6).
-
Infected seed and pollen. PSTVd is assumed to have spread among potato germplasm collections all over the world via infected true seed. Once present in a germ bank, the viroid can be transmitted to other (wild) potato plants either mechanically or by pollen exchange. Seed is also a potential source of infection for other crops such as tomato and pepper that are propagated by seed.
-
Aphid transmission. This route of transmission requires the source plant to be infected by both
Potato leafroll virus (PLRV) and PSTVd, thereby limiting the number of potential infection sources. PSTVd is assumed to be encapsidated by the viral coat protein; such encapsidation protects the viroid from digestion by micrococcal nuclease in vitro, suggesting that a similar protective effect may occur in vivo.

Figure 6 |
Epidemiology
The epidemiology of PSTVd is complicated because of the large number of host species and multiple potential transmission routes. For vegetatively propagated crops such as potato and ornamentals, the main mode of spread is propagation by infected seed potatoes and/or cuttings. Transmission by contaminated machinery or aphids carrying both PSTVd and PLRV may result in further spread.
Contaminated seed is an important source of infection for crops such as tomato that are grown from seed, but other routes of transmission should also be considered. For example, comparison of the respective nucleotide sequences of PSTVd indicated connections between PSTVd infections in several lots of tomato and other infections in lots of the ornamental
S. jasminoides. Because
S. jasminoides, or its vegetatively propagated progeny, is kept in the greenhouse year-round, it can serve as a persistent source of inoculum for tomato crops, which are removed from the greenhouse after every production cycle. This indicates that
S. jasminoides was the original source of infection in tomato and not
vice versa.
Disease Management
Disease management can be divided into two parts:
prevention of infection and
viroid eradication.
Prevention of infection includes all measures to prevent the introduction of PSTVd into a specific crop. It is very important to start a new cultivation with viroid-free planting material (tubers, seeds or plants). PSTVd is considered a quarantine 'organism' in many countries, and therefore, governmental measures to prevent introduction of PSTVd with plants from other countries will often be applied. Certification schemes including testing may be required to provide further guarantees that the planting material is free from PSTVd.
In addition to the use of healthy planting material, it is also important to prevent viroid introduction via human activities. Because PSTVd is mechanically transmissible, it can be introduced into potential host plants via the hands, clothes, or equipment used by people working in or visiting the greenhouse. The use of disposable gloves and specific clothing and equipment that stays inside a greenhouse compartment may prevent PSTVd introduction into greenhouse-grown crops. Furthermore, increasing the number of plants species grown in a greenhouse compartment increases the risk of introduction of PSTVd. Growers should either grow a single crop or they should separate different crops and lots, preferably in different compartments. Because PSTVd can be transmitted by aphids, planting PLRV-free seed potatoes and controlling aphid populations also contributes to the management of PSTVd in potato crops.
Viroid eradication is based on destruction of PSTVd-infected plants and thorough cleaning of equipment and greenhouses where infected plants have been grown. All infected plants together with those from an adequate buffer zone should be destroyed. In case of field-grown potatoes, crop rotations involving non-PSTVd host species help eliminate infected volunteer plants. In case of symptomless infections such as those commonly observed in ornamentals, all plants in the lot should be destroyed. Any rock wool or plastic used to cover the soil should also be removed from the greenhouse and destroyed. Ideally, all material slated for disposal should be transported in a closed container to an incinerator; alternatively, such material can be taken to a refuse dump and covered with a layer of soil.
When PSTVd is identified in a greenhouse-grown crop, all parts of the greenhouse should be thoroughly cleaned, preferably using a steam cleaner and a scrub brush for parts that are difficult to clean (Figure 7). A regular acid treatment can be used for watering tubes and drippers. After cleaning the greenhouse and associated equipment, application of a disinfectant completes the eradication procedure. When cultivation of crops susceptible to PSTVd infection resumes, extra monitoring for PSTVd symptoms and/or testing are advisable.

Figure 7A |

Figure 7B |
Significance
Economic significance During the last century, serious infestations of PSTVd affecting both commercial potato crops and potato breeding material have been reported in several parts of the world (Figure 8). For North America, total yield losses due to PSTVd infection were calculated to be approximately 1%, a figure which may have been low as a percentage but was still significant in absolute terms because of the large scale of potato production. Extensive testing and certification of seed potatoes have currently eliminated PSTVd or at least substantially reduced its impact on potato production in most countries. This success could only have been achieved because new PSTVd introductions in potato crops are rare. PSTVd occasionally also causes serious infections in tomato. Although the impact for individual grower may be severe, the overall significance in this crop seems limited as the yearly number of registered outbreaks is very low.
Scientific significance Potato spindle tuber disease was first described in 1922, and its infectious nature was demonstrated soon thereafter. These reports - only 25 years after Beijerinck's demonstration of the subcellular nature of tobacco mosaic virus (TMV) – appeared at a time when the viral nature of many diseases affecting potatoes and other crop plants was just beginning to be appreciated. Indeed, until 1967 potato spindle tuber disease was universally believed to be caused by a virus.
In a 1971 landmark publication, T.O. Diener demonstrated that the causal agent of spindle tuber disease differs in several fundamental respects from a conventional virus. These differences include i) a pathogen that exists in vivo as a single species of unencapsidated (no coat protein), low molecular weight RNA, ii) the absence of virus-like particles in infected tissue, and iii) the ability of the infectious RNA to replicate without the assistance of a helper virus. The term "viroid" was introduced by Diener to highlight these differences from conventional viruses. The small
satellite RNAs associated with several RNA viruses are similar in size to PSTVd, but all require the presence of their respective helper viruses for replication.

Figure 8A |

Figure 8B |
In 1978, another milestone in plant pathology was reached when the complete nucleotide sequence of PSTVd was determined using a combination of classical (i.e., non-cDNA based) RNA sequencing techniques. As what has been termed the "pioneering phase" of viroid research came to a close, several other important diseases in addition to spindle tuber were shown to be caused by viroids; i.e., citrus exocortis, chrysanthemum stunt, and avocado sunblotch.
Determination of the nucleotide sequence of PSTVd ushered in an era of viroid molecular biology that continues to the present day. The first studies of PSTVd replication involving cloned viroid cDNAs appeared in 1981, and the demonstration that greater-than-full-length PSTVd cDNAs are potentially infectious two years later opened the way for systemic investigation of PSTVd replication, movement, and disease induction using site-directed mutagenesis. Of the many subsequent investigations involving infectious PSTVd cDNAs, two studies in particular illustrate how PSTVd has been used to identify previously unsuspected plant/pathogen interactions. In 1994 (well before the full significance of RNA silencing was appreciated), Wassenegger and colleagues showed that integration of a PSTVd transgene capable of initiating autonomous RNA-RNA viroid replication into the nuclear genome of tobacco resulted in
de novo methylation of the transgene, thereby suggesting the existence of a mechanism by which cellular RNAs could target their own genes in a sequence-specific manner. With respect to transport of viroids and other RNAs from cell to cell in higher plants, Ding and colleagues have recently used a combination of site-directed mutagenesis and in situ hybridization to identify a specific structural motif in PSTVd that controls its ability to move across cell and tissue boundaries in the vascular system.
Selected References
Diener, T.O. 1971. Potato spindle tuber "virus" IV. A replicating, low molecular weight RNA.
Virology 45:411-428.
Diener, T.O. 1987. Potato spindle tuber. In: The Viroids (T.O. Diener, ed.), pp. 221-233. Plenum Press, New York.
Diener, T.O. 2003. Discovering viroids – a personal perspective.
Nature Reviews Microbiology. 1:75–80.
Flores, R., J.W. Randles, R.A. Owens, M. Bar-Joseph, and T.O. Diener. 2005. Subviral agents: Viroids. In: Fauquet, C.M., M.A. Mayo, J. Maniloff, U. Desselberger, and L.A. Ball (eds.), Virus Taxonomy. Eighth Report of the International Committee on Taxonomy of Viruses (pp 1147-1161) Elsevier Academic Press, San Diego, USA.
Gross, H.J., H. Domdey, C. Lossow, P. Jank, M. Raba, H. Alberty, and H-L. Sänger. 1978. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature 273:203–208.
Hadidi, A., R. Flores, J.W. Randles, and J.S. Semancik (editors). 2003. Viroids. CSIRO Publishing, Collingwood (Australia), 370 pp.
Hammond, R.W. and R.A. Owens. 2006. Viroids. New and Continuing Risks for Horticultural and Agricultural Crops. APSnet Feature article,
http://admin.apsnet.org/publications/apsnetfeatures/Pages/Viroids.aspx
Owens, R.A. 2007. Potato spindle tuber viroid: The simplicity paradox resolved? Molecular Plant Pathology 8:549-560.
Qi, Y., T. Pelissier, A. Itaya, E. Hunt, M. Wassenegger, and B. Ding. 2004. Direct role of a viroid RNA motif in mediating directional RNA trafficking across a specific cellular boundary. Plant Cell 16:1741-1752.
Riesner, D. 1987. Physical-chemical properties: Structure formation. In: The Viroids (T.O. Diener, ed.), pp. 63-98. Plenum Press, New York.
Schultz, E.S. and D. Folsom, 1923. Transmission, variation, and control of certain degeneration diseases of Irish potatoes. Journal of Agricultural Research 25:43-118.
Verhoeven, J.Th.J., C.C.C. Jansen, M. Botermans, and J.W. Roenhorst. 2010. Epidemiological evidence that vegetatively-propagated, solanaceous plant species act as sources of Potato spindle tuber viroid inoculum for tomato. Plant Pathology 59, 3-12.
Wang, M-B., X-Y Bian, L-M. Wu, L-X. Liu, N.A. Smith, D. Isenegger, R-M. Wu, C. Masuta, V.B. Vance, J.M. Watson, A. Rezaian, E.S. Dennis, and P.M. Waterhouse. 2004. On the role of RNA silencing in the pathogenicity and evolution of viroids and viral satellites. Proceedings of the National Academy of Sciences. USA 101:3275–3280.
Wassenegger, M.E., S. Heimes, L. Riedel, and H.L. Sänger. 1994. RNA-directed de novo methylation of genomic sequences in plants. Cell 76:567-576.
