Kemmitt, G. 2002. Early blight of potato and tomato. The Plant Health Instructor. DOI: 10.1094/PHI-I-2002-0809-01
Updated 2013.
Early blight
Alternaria solani
Tomato (Lycopersicon esculentum), Potato (Solanum tuberosum)
Author
Greg Kemmitt
Dow AgroSciences, 9330 Zionsville Road, Indianapolis, IN, USA
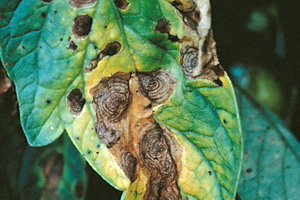
Alternaria solani on tomato foliage.
(Courtesy W.R. Stevenson)
Symptoms
Symptoms of early blight occur on fruit, stem and foliage of tomatoes and stem, foliage and tubers of potatoes. Initial symptoms on leaves appear as small 1-2 mm black or brown lesions and under conducive environmental conditions the lesions will enlarge and are often surrounded by a yellow halo (Figures 2 and 3). Lesions greater than 10 mm in diameter often have dark pigmented concentric rings. This so-called “bullseye” type lesion is highly characteristic of early blight (Figure 4). As lesions expand and new lesions develop entire leaves may turn chlorotic and dehisce, leading to significant defoliation. Lesions occurring on stems are often sunken and lens-shaped with a light center, and have the typical concentric rings (Figure 5). On young tomato seedlings lesions may completely girdle the stem, a phase of the disease known as “collar rot,” which may lead to reduced plant vigor or death.

Figure 2 |

Figure 3 |

Figure 4 |

Figure 5 |
Infection of both green and ripe tomato fruit normally occurs through the calyx with lesions sometimes reaching a considerable size (Figure 6). The lesions appear leathery and may have the characteristic concentric rings. Infected fruit will frequently drop prematurely. Symptoms on potato tubers are characterized by sunken, irregular lesions (Figure 7), which are often surrounded by a raised purple border. Beneath the surface of the lesion the tuber tissue is leathery or corky with a brown discoloration. Early blight lesions on tubers tend to be dry and are less prone to invasion by secondary organisms than lesions of other tuber rots. After prolonged storage severely diseased tubers may become shriveled.

Figure 6 |
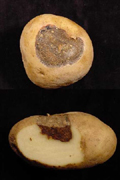
Figure 7 |
Pathogen biology
The causal pathogen of early blight is the fungus Alternaria solani. There is no known sexual stage and hence it is classified as a Deuteromycete. The genus Alternaria is a large and important group of pathogenic fungi, which cause a significant number of important diseases. The fungus is readily cultured on artificial media such as V8 juice where it produces a deeply pigmented gray/black hairy colony. The mycelium is haploid and septate, becoming darkly pigmented with age. Sporulation in culture can be stimulated by exposure to fluorescent light. The asexual conidia are borne singly or in a chain of two on distinct conidiophores. The beaked conidia normally possess 9–11 transverse septae (Figure 8). Morphological and pathogenic variability among isolates of A. solani has given rise to claims of the existence of races, although this remains unproven.
 Figure 8
Figure 8Disease Cycle and Epidemiology
Alternaria solani overwinters primarily on infected crop debris. The dark pigmentation of the mycelium increases resistance to lysis which extends the survival time in the soil to several years. Thick-walled chlamydospores have been reported, but they are found infrequently. In mild climates the pathogen can survive from season to season on volunteer tomato and potato plants as well as other weedy Solanaceous hosts such as horsenettle and nightshade.
Warm, humid (24-29°C/ 75-84°F) environmental conditions are conducive to infection. In the presence of free moisture and at an optimum of 28-30°C (82-86°F), conidia will germinate in approximately 40 min. Desiccated germ tubes are able to renew growth when re-wetted, and, hence, infection can occur under conditions of alternating wet and dry periods. Germ tubes penetrate the leaf epidermis directly or enter through stomata. Infection of potato tubers usually occurs through wounds in the tuber skin inflicted during harvest. Wet conditions at harvest provide a favorable environment for spore germination as well as causing swollen lenticels on the tubers which are easily invaded.
Time from initial infection to appearance of foliar symptoms is dependent on environmental conditions, leaf age, and cultivar susceptibility. Early blight is principally a disease of aging plant tissue. Lesions generally appear quickly under warm, moist conditions on older foliage and are usually visible within 5-7 days after infection.
A long wet period is required for sporulation but it can also occur under conditions of alternating wet and dry periods. Conidiophores are produced during wet nights and the following day light and dryness induce them to produce spores, which emerge on the second wet night.
Secondary spread of the disease results from conidia being dispersed mainly be wind and occasionally by splashing rain or overhead irrigation. Early blight is considered polycyclic with repeating cycles of new infection. This is the period when the disease has the potential to spread rapidly and build up to damaging levels in the crop.
Disease Management
Cultural practices
In many cases employing sound cultural practices that maintain potato and tomato plants in good health will keep early blight losses below economic levels. Because the pathogen over winters on infected crop debris, in-field sanitation procedures that reduce initial inoculum in subsequent crops are beneficial. Consideration should be given to removing potentially infected material such as decaying vines and fruits from the vicinity of production fields. Controlling volunteers and weeds, such as nightshade and horsenettle which serve as alternative hosts for the disease, prior to planting the new crop will help to reduce the risk of transmission of disease. Ensuring seed or transplants are pathogen free before placing out in the field and rotating fields to a non susceptible host crop will also help to reduce buildup of inoculum in the soil. Optimal tuber maturity is the most important factor for control of tuber infection. Tubers harvested before maturing are susceptible to wounding and infection. Tuber infection can be reduced by careful handling during harvest to minimize wounding as well as avoiding harvesting during wet conditions if possible. Tubers should be stored at 50 to 55 F, at high relative humidity and with plenty of aeration to promote wound healing which will reduce the amount and severity of tuber infections that develop in storage.
 Figure 9
Figure 9During the season, overhead irrigation schedules should minimize the duration of leaf wetness in the crop (Figure 9). Avoid irrigation in cool, cloudy periods or late in evening when foliage may stay wet for extended periods. Selecting fields with good drainage and an absence of natural impediments to air flow over the crop, e.g. rows of trees, will reduce periods of leaf wetness. Maintenance of adequate soil fertility levels is also critical for managing early blight. The disease is often associated with crops suffering from a lack of nitrogen, particularly towards the end of the growing season on older senescing foliage. Management of other diseases such as Verticillium wilt will reduce plant stress and hence, early blight severity.
Resistant cultivars
Complete resistance to early blight does not exist in commercial potato or tomato cultivars. Using wild Lycopersicon species which show a high degree of resistance in breeding programs has led to the release of a number of cultivars of potato and tomato with a degree of resistance to early blight. Apparent levels of resistance are often correlated with plant age. Immature potato and tomato plants are relatively resistant to early blight but, after tuber and fruit initiation, susceptibility increases gradually, and mature plants are very susceptible to the pathogen.
 Figure 10
Figure 10Chemical control
Fungicides with protectant and curative properties are registered for use against early blight on tomato and potato (Figure 10). The cheaper protectant fungicides such as mancozeb and chlorothalonil are the foundation of most early blight management programs. These fungicides must be reapplied every 7-10 days to provide protection of new growth as well as to counter the effects of weathering which progressively removes the chemical from the leaf surface. Advantages of these types of products include their reliable efficacy and multi-site mode of action, which reduces the risk of resistant isolates developing in the pathogen population; therefore, they are useful as tank mix partners or used in rotation with other fungicides. Disadvantages include the need to apply regularly and their relatively high use rates.
The so-called Quinone Outside Inhibitors (QoI) class of fungicides (FRAC code #11) which inhibit fungal respiration are highly active against Alternaria species. Molecules from this very important class of fungicides which are registered for Alternaria control in potato and or tomato include azoxystrobin, pyraclostrobin, trifloxystrobin, fenamidone and famoxidone. In general QoIs are readily taken up into the plant tissue and work preventively to stop infection by inhibiting spore germination. They are weakly curative and use rates are considerably lower than the traditional protectant products, although cost per acre is typically higher. QoIs are high-risk fungicides with respect to resistance development, and isolates of A. solani which possess the F129L mutation have been isolated from the field. These isolates show significantly reduced levels of sensitivity to the QoI fungicides ( Pasche and Gudmestad, 2008). Isolates of A. solani bearing the G143A mutation which confers high levels of resistance to QoIs have also been detected in routine monitoring in Europe. The Fungicide Resistance Action Committee (FRAC) discourages the use of QoIs in a curative manner and recommends that this fungicide class should make up no more than six applications or 50% of the total spray program in a single season providing the material is applied with another fungicide having an alternative mode of action, either as a tank mixture or in a co-formulated product. If the QoI is applied solo to the crop then the grower should not exceed 4 sprays or 33% of the total spray program.
Growers also have new weapons in their chemical arsenal for control of early blight in the shape of fluxapyroxad, fluopyram and penthiopyrad which are all succinate dehydrogenase inhibitors (SDHIs) -FRAC code #7. SDHIs are also inhibitors of fungal respiration although they bind to a different target site than the QoIs and hence are not cross resistant to the latter. These materials are new in the potato and tomato market in 2012 and will give growers a welcome additional mode of action for managment of early blight. As single-site inhibitors they are consider by FRAC as at medium to high risk of resistance development and will require careful management of the resistance risk via appropriate tank mixing and alternation with other effective modes of action.
Timing of fungicide sprays relative to environmental conditions and subsequent potential for disease development is critical if good control is to be attained. Use of disease forecasting programs such as FAST in tomatoes and P-DAY in potatoes to correctly time application of sprays as well as thorough scouting of fields increases efficacy of the fungicide products as well as helping to cut down on unnecessary applications and costs. (Figures 11 and 12).

Figure 11 |

Figure 12 |
Significance
By the standards of modern agriculture the tomato and potato are relatively recently adopted crops. Wide scale cultivation gained prominence largely in the 19th century some 300 years after their introduction into Europe from the Andean region of South America. The tomato was initially considered a horticultural crop, but the development of the food processing industry and its appetite for large quantities of cheaply produced tomatoes led to the development of large-scale field production of tomatoes particularly in the US. Worldwide production of this crop in 2011 was approximately 159 million metric tonnes. The potato is a dietary staple in nearly all temperate countries with annual production worldwide in 2011 of around 375 million metric tonnes.
Early blight is distributed worldwide and essentially occurs wherever tomatoes and potatoes are grown. In the US early blight in tomatoes can be problematic east of the Rocky Mountains but is not usually a problem in the less humid inter mountain or Pacific regions. If left uncontrolled the disease may cause severe defoliation, resulting in reduced fruit size and number. In potatoes the disease occurs in most production areas to some degree every year although it has a significant effect on yield only when frequent wetting of the foliage favors early, rapid symptom development.
Estimating total annual crop losses to any one particular disease is difficult to do accurately. Values in the literature for measured crop losses due to early blight in unsprayed fields vary enormously from 5 – 78%. Precise figures for total expenditure on fungicides for control of early blight is also difficult to arrive at due to the fact that it is just one of a complex of tomato/potato pathogens which are normally controlled with the same products. Best estimates suggest annual expenditure globally on fungicides for control of Alternaria spp. is around $32 million in tomatoes and $45 million in potatoes.
Selected References
Christ, B.J. and S.A. Maczuga. 1989. The effect of fungicide schedules and inoculum levels on early blight severity and yield of potato. Plant Dis. 73: 695-698.
Gleason, M.L., A. A. MacNab, R.E. Pitblado, M.D. Ricker, D.A. East, and R.X. Latin. 1995. Disease warning systems for processing tomatoes in eastern North America: Are we there yet? Plant Dis. 79: 113-121.
Holley, J.D., R. Hall, and G. Hofstra. 1985. Effects of cultivar resistance, leaf wetness duration and temperature on rate of development of potato early blight. Can. J. Plant Sci. 65: 179-184.
Stevenson, W.R., R. Loria, G.D. Franc, and D.P. Weingartner. 2001. Compendium of Potato Diseases. Second Edition. APS Press, St. Paul, MN.
Jones, J.B., J.P. Jones, R.E. Stall, and T.A.Zitter. 1991 Compendium of Tomato Diseases. APS Press, St. Paul, MN.
Madden, L., S.P. Pennypacker, and A.A. MacNab. 1978. FAST, a forecast system for Alternaria solani on tomato. Phytopathology 68: 1354-1358.,
Pscheidt, J.W. and W.R. Stevenson. 1986. Early blight of potato and tomato: A literature review. University of Wisconsin, Cooperative Extension Service Publication No. R3376.
Pscheidt, J.W. and W.R. Stevenson. 1988. The critical period for control of early blight (Alternaria solani) of potato. Am. Potato J. 65: 425-438.
Rotem, J. 1994. The Genus Alternaria: Biology, Epidemiology and Pathogenicity. APS Press, St. Paul, MN.
Stevenson, W.R. 1993. Management of early blight and late blight. Pages 141 -147. In Potato Health Management. Rowe, R.C. (ed), APS Press, St. Paul, MN.
Soltanpour, P.N. and M.D. Harrison. 1974. Interrelations between nitrogen and phosphorus fertilization and early blight control of potatoes. Am. Potato J. 51: 1-7.
Vloutoglou, I and S.N. Kalogerakis. 2000. Effects of inoculum concentration, wetness duration and plant age on development of early blight (Alternaria solani) and on shedding of leaves in tomato plants. Plant Path. 49: 339-345.
