TeBeest, D.O., C. Guerber and M. Ditmore. 2007. Rice blast.
The Plant Health Instructor. DOI: 10.1094/PHI-I-2007-0313-07.
Reviewed 2012.
Rice Blast
Magnaporthe oryzae (anamorph:
Pyricularia oryzae)
Rice (Oryza sativa).
Authors
David O. TeBeest,
Claudia Guerber, and
Michael Ditmore
University of Arkansas
|
Figure 1 |
Figure 2 |
Figure 3 |
Hundreds of millions of people world-wide depend on rice as a staple food (Figures 1, 2). A crop failure, for any reason, poses a real threat of starvation. Rice blast, caused by a fungus, causes lesions (Figure 3) to form on leaves, stems, peduncles, panicles, seeds, and even roots. So great is the potential threat for crop failure from this disease that it has been ranked among the most important plant diseases of them all. Other grasses, including crabgrass, are infected with closely related fungi (Magnaporthe grisea, Magnaporthe poae, Magnaporthe rhizophila and
Magnaporthe salvinii), which cause nearly identical symptoms on their respective hosts.
Symptoms and Signs
The symptoms of rice blast include lesions that can be found on all parts of the plant, including leaves, leaf collars, necks, panicles, pedicels, and seeds. A recent report shows that even roots can become infected. However, the most common and diagnostic symptom, diamond shaped lesions, of rice blast occur on the leaves, whereas lesions on the sheaths are relatively rare.
Rice leaves. The symptoms on leaves may vary according to the environmental conditions, the age of the plant, and the levels of resistance of the host cultivars (Figure 4). On susceptible cultivars, lesions may initially appear gray-green and water-soaked with a darker green border and they expand rapidly to several centimeters in length. On susceptible cultivars, older lesions often become light tan in color with necrotic borders. On resistant cultivars, lesions often remain small in size (1-2 mm) and brown to dark brown in color.
Rice collars. The collar of a rice plant refers to the junction of the leaf and the stem sheath. Symptoms of infection of the collars consist of a general area of necrosis at the union of the two tissues (Figure 5). Collar infections can kill the entire leaf and may extend a few millimeters into and around the sheath. The fungus may produce spores on these lesions.
Rice necks and panicles. The neck of the rice plant refers to that portion of the stem that rises above the leaves and supports the seed head or panicle. Necks are often infected at the node by the rice blast fungus and infection leads to a condition called rotten neck or neck blast (Figure 6). Infection of the necks can be very destructive, causing failure of the seeds to fill (a condition called blanking) or causing the entire panicle to fall over as if rotted. The rice blast fungus can also infect the panicles as the seeds form (Figure 7). Lesions can be found on the panicle branches, spikes, and spikelets. The lesions are often gray brown discolorations of the branches of the panicle, and, over time, the branches may break at the lesion.
Rice seeds. The fungus has often been isolated from the pedicels of the seeds. Seeds are not produced when pedicels become infected, a condition called blanking. Symptoms of rice blast on seeds themselves consist of brown spots, blotches (Figure 8), and occasionally the classic diamond-shaped lesion often seen on leaves. The process and the time during which infection of seeds by spores of the pathogen occurs has not been fully described but recent information shows that the fungus can infect seeds by infecting the florets as they mature into seeds, and it is believed that this is the main way seed infection develops.

Figure 4 |

Figure 5 |

Figure 6 |

Figure 7 |

Figure 8 |
Pathogen Biology
The fungus that causes rice blast is called
Magnaporthe oryzae (formerly
Magnaporthe grisea) (Figure 9). It is an ascomycete because it produces sexual spores (ascospores) in structures called
asci, and is classified in the newly erected family Magnaporthaceae. The asci are found within specialized structures called
perithecia. The mycelium of
M. oryzae is septate and the nuclei within the mycelium and spores of this fungus are haploid.
Sexual Reproduction
The sexual, or teleomorphic, stage of the rice blast pathogen can be produced in the laboratory if isolates of opposite mating type are paired, but has not been found in the field in the United States. As an ascomycete, it produces hyaline, fusiform shaped (spindle-shaped with tapering ends) ascospores with three septa. The asci are unitunicate. This fungus is considered to be heterothallic with a bipolar mating system (mating controlled by two different alleles at a single locus) with additional genes controlling the sexual cycle. Based on recent phylogenetic, molecular and morphological data, isolates of the fungus from rice and closely related isolates from other grasses like
Eragrostris curvula, Eleusine coracana, Lolium perenne, and
Setaria spp. are taxonomically described as
Magnaporthe oryzae, while isolates from
Digitaria sanguinalis (crabgrass) are distinct and should be described as
Magnaporthe grisea.
Asexual Reproduction
The asexual stage of
Magnaporthe oryzae is described by the name
Pyricularia oryzae (formerly called
P. grisea) and it is the most common spore form of the fungus in the USA (Figure 10). These spores, called conidia, are produced abundantly on lesions and in culture on specialized stalks, called conidiophores. The conidia are usually three-celled, and produced on the apex of a conidiophore. Sporulating colonies on agar plates may take on a fleecy grayish appearance.
Under favorable conditions, the fungus sporulates in the center of the lesions on susceptible cultivars (Figure 11). It can also sporulate on seed lesions (Figure 12). It rarely sporulates on the most resistant cultivars. Spores are produced on infected leaf, collar, panicle, and seed, on conidiophores that extend beyond lesion surfaces; the conidiophores and spores en-masse may give the lesions a dusty gray appearance. Conidia are produced after several hours of high humidity and are easily released or liberated near mid-day, especially under windy conditions.
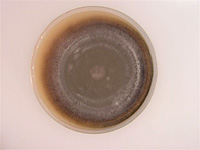
Figure 9 |
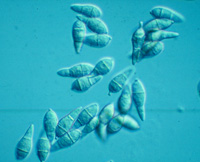
Figure 10 |

Figure 11 |
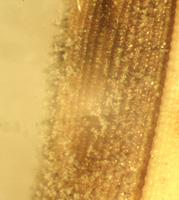
Figure 12 |
The Infection Process
Infection of rice occurs when conidia are deposited on rice tissues, and germinate by producing a germ tube and an
appressorium. The appressorium is a melanized structure, and from it develops an infection peg which penetrates the tissue. After penetration, the primary infection hypha grows rapidly and ramifies within susceptible tissues. Growth within tissues of resistant cultivars is often inhibited.
Disease Cycle and Epidemiology
Plant diseases are often severe during periods of warm temperatures and high moisture. In the United States, rice is grown in Arkansas, California, Florida, Louisiana, Mississippi, Missouri, and Texas (Figure 13). In the United States, rice is planted mechanically (Figure 14) as seed, while in many other countries, rice seedlings are grown in beds and transplanted manually or by machine. Throughout the world, rice is normally grown partially submerged in water in paddies, although in some regions rice is grown as upland rice in much the same way as wheat. Generally, rice blast is favored by moderate temperatures (24C) and periods of high moisture that are 12 hours or longer, conditions readily attainable in flooded rice fields.
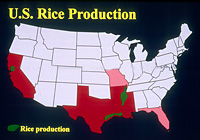
Figure 13 |

Figure 14 |
The overwintering sources of spores that comprise the primary inoculum consist of grasses, volunteer plants, infested refuse (Figure 15), and infested seed on the soil surface after mechanical planting (Figure 16). Infested seeds left on the soil surface can readily produce spores of
P. oryzae for more than several weeks after planting, well after seedlings have emerged (Figure 17). In the greenhouse, the fungus also sporulates on the dead or dying coleoptiles of plants grown from infected seeds.

Figure 15 |

Figure 16 |
Figure 17 |
Spores produced as the primary inoculum on the overwintering tissues produce the initial infections on young seedlings when the spores that are deposited on leaves, germinate and invade leaf tissues. Disease severity is often correlated with the amount of infested material (Figure 18). For example, in Arkansas, the first visible lesions often appear approximately 45 to 55 days after planting, but the disease is much more severe in plots receiving the highest amounts of primary inoculum found on infested seeds. Lesions on the young seedlings appear within a few days after infection. These secondary lesions produce more spores and these spores are readily wind disseminated to nearby healthy leaf tissues. The secondary cycles can be repeated many times during the growing season, with the potential for very high amounts of disease within the crop. The number of cycles and the number of spores that are produced on each individual lesion can be influenced by many factors, including the temperature, rainfall, the depth of the water in the paddy, the amount of nitrogen used to fertilize the rice, and the level of genetic resistance in the cultivar that is infected (Figure 19). Generally, the leaf phase of the disease is most severe when daily temperatures are moderate, and when rice is over-fertilized, or if it is being grown in flood waters below the recommended depths. Under these conditions conducive to disease, very high incidences of disease have been recorded (Figure 19) on susceptible cultivars such as Newbonnet and RT7015 in comparison to cultivars genetically resistant to the fungus.
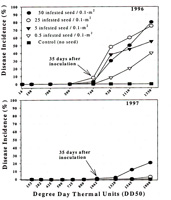
Figure 18 |
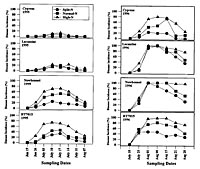
Figure 19 |
The amount of disease at the end of the vegetative phase of the growing season influences the amount of disease during the reproductive phase. Spores produced near the end of the growing season may infect the collar of the flag leaf producing symptoms called collar rot. They may also infect the neck when it emerges from the infected collar upon which the head will be supported to produce a condition called rotten neck or neck blast. Yield losses are significantly correlated with the extent of rotten neck. Research has shown a 0.5% loss in yield for every 1% of rotten neck. Infections of the neck are generally considered the most deleterious phase of the disease because infection at this location can reduce seed set on the entire panicle. In addition, the fungus can infect the panicle, the branches on the panicle, and the peduncles upon which the seeds are carried on these branches. Finally, new information shows that the seed also can be infected. Infections of the neck, panicle and branches of the panicle are usually relatively nondescript grayish discolorations of the tissues.
Disease Management
The successful management of rice blast results from a comprehensive series of recommendations that employ several different management strategies involving techniques within each strategy as outlined below.

Figure 20 |

Figure 21 |
Cultural strategies to manage this disease include useful techniques that rice producers are urged to follow. Crop rotation is one simple and effective technique that is highly recommended simply because it provides a mechanism that separates viable spores in crop residue (Figure 15) from the newly emerging seedlings. A second technique that rice producers are urged to consider is to fertilize each of the different cultivars properly. Overuse of nitrogen fertilizers, as shown above, increases the amount of rice blast in their fields while often failing to significantly increase yields. A third technique that is often overlooked or difficult to employ in some fields is maintaining a proper flood level for the rice to grow (Figure 20). Rice blast is known to be more severe in fields or parts of fields in which the water in paddies falls below recommended levels. Thus, rice blast is often severe in fields that were not rotated, are under-irrigated, and are over-fertilized (Figure 21). Finally, using high quality and disease-free seed is always highly recommended because infested seeds left on the soil surface provide inoculum from which epidemics develop.
Genetic resistance is a strategy that has long been the mainstay of successful rice production in the United States, even though the fungus has shown genetic variability for virulence. In the United States, many rice cultivars contain genes that confer resistance to one or more of the individual races of the fungus found regionally, and many also contain a high level of resistance to the fungus that is conferred by many other genes. The major difficulty in controlling rice blast with genetic resistance is that there are many races of the pathogen, and cultivars containing a single gene conferring resistance to a specific race of the pathogen often become susceptible over time with the development of new races that can infect plants with that specific resistance gene. For example, a mutation of an 'avirulence' gene in the blast fungus that produces an elicitor that induced the expression of resistance in the host with the Pi-ta gene for resistance has led to the formation of a new race capable of infecting plants that carry that important resistance gene, simply because the plant did not recognize the new race of the pathogen. In this way, the new race of the fungus has eluded resistance, which means that the cultivars containing this important resistance gene are very susceptible to the new race, even though they remain resistant to the other races of the fungus.
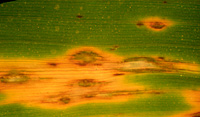
Figure 22 |

Figure 23 |
A third strategy that has long been viewed as a last resort for rice blast is the use of chemical fungicides to control the disease. There are two basic techniques that can be used to manage diseases with the chemical fungicide strategy. The first technique uses seed treatments to prevent infection of the seedlings after germination. The second technique uses fungicides to prevent infection of leaves and panicles during the growing season by making one or two applications of fungicides to the foliage to protect the panicles when they are emerging from the boot. This technique attempts to reduce the incidence of rice blast on the panicle necks and panicles. Both techniques have been used to manage rice blast but neither one is considered to be highly successful. In addition, the use of foliar fungicides applied by airplanes (Figure 24) can be very expensive.

Figure 24 |
Managing rice blast requires vigilance and careful integration of many strategies and techniques learned in all parts of the world by the individual rice producer. A single mistake can result in crop failure from this one important plant disease.
Significance of the Disease

Figure 25 |
Rice is the staple food crop for a large part of the human population in the world today. Rice blast is by far the most important disease of the many diseases that attack rice. It is found wherever rice is grown, it is always important, and it is always a threat. Failures of entire rice crops have resulted directly from rice blast epidemics. The challenge for research continues to be to produce high quality food, in ever-increasing amounts at a lower costs, all while in the presence of an unforgiving and unrelenting pathogen. All of the plant disease management strategies and techniques that have been generated through research have been brought to bear against rice blast, but often with limited success. Rice blast has never been eliminated from a region in which rice is grown, and a single change in the way in which rice is grown or in the way resistance genes are deployed can result in significant disease losses even after years of successful management. This disease is a model that demonstrates the seriousness, elusiveness, and longevity of some plant diseases. Rice blast has been widely studied throughout the world. Many investigators have considered it to be a model disease for the study of genetics, epidemiology, molecular pathology of host parasite interactions and biology. Recent advances in understanding the genes that govern the avirulence (resistance) and virulence (susceptibility) interactions have been made with rice blast, and each advance has helped us to understand how other plant diseases work. It is also important to note that the entire genomes of the rice blast fungus and rice have been sequenced and that
M. oryzae is the first plant pathogenic fungus to have its genome sequenced and released to the public.
Selected References
Bonman, J.M. 1992. Rice Blast.
In: Compendium of Rice Diseases. Eds. R.K. Webster and P.S. Gunnel. American Phytopathological Society Press. St. Paul, Minnesota. USA. Pages 14-18.
Couch, B.C. and L.M. Kohn. 2002. A multilocus gene genealogy concordant with host preference indicates segregation of a new species,
Magnaporthe oryzae from
M. grisea. Mycologia 94: 683-693.
Howard, R.J. and B. Valent. 1996. Breaking and entering: host penetration by the fungal rice blast pathogen. Annual Review of Microbiology 50: 491-512.
Jia, Y., S.A. McAdams, G.T. Bryan, H.P. Hershey and B. Valent. 2000. Direct interaction of resistance gene and avirulence gene product confers rice blast resistance. EMBO Journal 19: 4004-4014.
Long, D.H., F.N. Lee, and D.O. TeBeest. 2000. Effect of nitrogen fertilizer on disease progress on susceptible and resistant cultivars. Plant Disease 84: 403-409.
Long, D.H., J.C. Correll, F.N. Lee, and D.O. TeBeest. 2001. Rice blast epidemics initiated by infested rice grain on the soil surface. Plant Disease 85: 612-616.
Orbach, M.J., L. Farrall, J.A. Sweigard, F.G. Chumley, and B. Valent. 2000. A telomeric avirulence gene determines efficacy for the rice blast gene Pi-ta. Plant Cell 12: 2019-2032.
Rossman, A.Y., R. Howard and B. Valent. 1990.
Pyricularia grisea, the correct name for the rice blast disease fungus. Mycologia 82: 509-512.
Zhou, E., Y. Jia, P. Singh, J.C. Correll and F.N. Lee. 2007. Instability of the
Magnaporthe oryzae avirulence gene AVR-Pita alters virulence. Fungal Genetics and Biology. (In Press).
