Rice Sheath Blight
Rhizoctonia solani AG1-1A Kühn (Teleomorph: Thanatephorus cucumeris (A. B. Frank) Donk.)
Rice, soybean, bean, sorghum, corn, sugarcane, turfgrass, and weed hosts such as barnyard grass, crabgrass, and broadleaf signal grass.

R. solani infects rice leaf sheaths at the base of culms,
producing oblong, gray-brown, water-soaked lesions.
Rice sheath blight is one of the most economically significant rice diseases worldwide. This disease causes significant grain yield and quality losses. Yield losses of up to 50% have been reported under most conducive environments. Sheath blight is a soilborne disease caused by the fungus Rhizoctonia solani AG1-IA. The fungus belongs to the phylum Basidiomycota, family Ceratobasidiaceae.
Symptoms and Signs
Symptoms
Early symptoms usually develop on the leaf sheaths at or just above the water line as circular, oval or ellipsoid, water-soaked spots which are greenish-gray in color (Figure 1). As the disease progresses, they enlarge and tend to coalesce forming larger lesions with grayish white centers surrounded by tan to dark brown irregular borders or outlines. Infection can spread to leaf blades and cause irregular lesions with dark green, brown, or yellow-orange margins (Figure 2). The lesions can develop extensively and coalesce on partial or whole leaf blades, which may produce a rattlesnake skin pattern. These damaged tissues interrupt the normal flow of water and nutrients to the plant tissues above (leaves and panicles). As the plant approaches heading, the canopy becomes dense, creating a humid microclimate that is favorable for the rapid development of the disease. The disease may move up the plant and infect the flag leaves and panicles under severe conditions. The fungus can spread into the culms from early sheath infections and weaken the infected culms, resulting in the lodging and collapse of tillers. The damage caused by sheath blight ranges from partial infection of the lower sheaths with little impact on grain filling to the premature death of plants and lodging with a significant reduction in grain yield and quality.

Figure 1 |

Figure 2 |

Figure 3 |
Signs
Microscopic runner hyphae and pea-sized sclerotia are two signs of pathogen infection. The white, web-like hyphae (threads) of the fungus grow on the sheaths and leaves under favorable conditions and serve to spread the disease from leaf to leaf, causing infections of nearby plants (Figure 4). Sclerotia of the fungus are formed in the infected sheaths and leaves most typically at the boot to heading stages (Figure 5). Sclerotia are interwoven masses of fungal mycelia coated by impervious, hydrophobic layers secreted by the fungus. Sclerotia are white when formed and turn brown or dark brown. They are loosely attached, and easily dislodge from the plants at maturity. Sclerotia are relatively spherical, occur individually measuring 1-3 mm in diameter or coalesce forming larger masses.

Figure 4 |

Figure 5 |
Pathogen Biology
Sexual reproduction
Sexual reproduction is not common in R. solani under field conditions. The perfect stage of R. solani is Thanatephorus cucumeris. The perfect stage appears as a thin, mildew-like growth on soil, leaves and infected sheaths just above the ground line. This stage is usually formed under high humidity and produces basidia with four sterigmata each bearing one basidiospore. Very little is known about the role of this stage in the natural development of sheath blight.
Asexual reproduction
R. solani does not produce any asexual spores or conidia. The fungus infects plants by vegetative mycelia or germinating sclerotia producing hyphal threads. Mycelium is white when young but turns yellowish or light brown with age (Figure 6), produces long multinucleate cells that grow approximately at right angles to the main hypha with a slight constriction at the junction of main hypha and branches (Figure 7). Hyphal cells are separated by septum containing a pore which allows movement of mitochondria, nuclei, and cytoplasm from cell to cell.
Figure 6 |
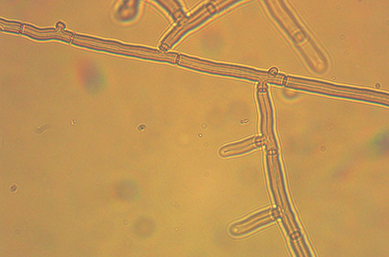
Figure 7 |
Disease Cycle and Epidemiology
Sclerotia and mycelia in infected plant debris are two primary sources of inoculum. During the cropping season or at harvest, sclerotia fall on the ground and serve as survival structures from one cropping season to other. They survive for long periods in the soil, with up to 2 years in temperate rice production areas, and frequently accumulate in the field over time. Field water movement and irrigation support the dispersal of sclerotia and infected plant debris. Initial infections start with a sclerotium or a piece of infected debris floating on the water surface and coming in contact with the sheath. The fungus gets attracted to the chemical stimuli released by the rice host. Germinating sclerotia or mycelia in debris penetrate the plant tissue either by means of natural openings or by specialized infection structures called appressoria or infection cushions. The fungus also produces extracellular enzymes that degrade plant cell walls to facilitate colonization. Once the fungus penetrates and colonizes the plant tissue, symptoms are initiated. The fungus grows upwards on the plant, penetrates, and infects upper leaf sheaths, leaf blades, and panicles (Figure 8). The fungus spreads in the field by growing its runner hyphae from tiller to tiller, from leaf to leaf, and from plant to plant, resulting in a circular pattern of damage. The infection spreads most quickly when susceptible varieties are grown under favorable conditions such as warm temperature (28 to 32°C), high humidity (95% or above), and dense stands with a heavily developed canopy. The disease frequently starts during the late tillering to joint elongation stages of plant growth and becomes more aggressive as the rice plant shifts to the panicle differentiation (reproductive) stage. Sheath blight also infects weed hosts and causes similar symptoms. Sclerotia are produced on the surface of infected tissues of rice and weed hosts and survive in the soil between crops. In addition, the fungus also infects other crops including soybeans, sorghum, corn, and sugarcane, increasing the inoculum in the soil. Factors that favor the development of sheath blight include the use of highly susceptible semi-dwarf rice varieties, short rotations with non-host crops, overuse of nitrogen fertilizer, and increased stands that create favorable microenvironments in the canopy.
Disease Management
An integrated management approach, consisting of resistant or moderately resistant varieties, sound cultural practices, and foliar fungicide application, is ideal for effective and economic control of sheath blight and associated yield losses. Varietal susceptibility, field disease history, timely scouting for the presence of the disease, and local weather conditions that favor the disease are among the critical factors to be considered for making effective disease management decisions. Sheath blight develops at a rapid pace under favorable conditions. The disease should be monitored on a regular basis starting from the panicle differentiation stage until heading. Infection after heading may cause insignificant economic losses due to sheath blight.
Cultural Practices
Varietal selection: Rice variety selection is the first important step towards reducing crop yield losses due to the disease. At present, there are no rice varieties with complete resistance against sheath blight. However, rice varieties with different levels of resistance are available. In general, most of the hybrid varieties are more resistant to the disease than most of the inbred varieties. Medium-grain varieties have more resistance against sheath blight than most of the long-grain varieties. Therefore, selecting a rice variety that is less susceptible or moderately resistant to sheath blight is the most effective way to reduce the damage caused by the disease.
Field sanitation: Levels of inoculum can be reduced by destroying weed hosts and other collateral hosts that could harbor sclerotia. However, this approach is not very effective or not feasible for management of sheath blight.
Management practices to avoid dense canopy: High seeding rate and overuse of nitrogen fertilizer usually increase stand and induce excessive vegetative growth and canopy density, creating a moist microclimate favorable for disease development. Therefore, avoiding high seeding rates and excessive application of fertilizers, especially nitrogen, can reduce the damage caused by sheath blight.
Crop rotation: Continuous rice or rotation with alternate hosts of the fungus such as soybeans increases inoculum in field soils. Fallow periods, along with efforts at reducing the inoculum by destroying collateral and weed hosts that could harbor sclerotia are viable management practices.
Chemical Control
Fungicides can be one of the most effective tools for control of sheath blight. In the United States, several fungicides, including azoxystrobin, provide excellent control of this disease. A single fungicide application is currently recommended to reduce production costs and maximize production returns. The timing of the application is critical for effective control of sheath blight. The disease should be scouted and monitored periodically during the development of the rice crop through heading. The application should be made during the growth stage between panicle differentiation plus five days and heading when the disease level reaches the economic threshold level. In many situations, foliar fungicide applications may be economically justified for reducing losses from sheath blight if 1) disease pressure is sufficiently high; 2) susceptible rice varieties are grown; 3) the crop has a high yield potential in the absence of sheath blight; and 4) environmental conditions are favorable for the disease to spread to the upper parts of the rice plant. The purpose of fungicide applications is to suppress sheath blight vertical development and reduce the grain yield and quality loss caused by the disease. Caution should be taken not to overuse fungicides with a single mode of action as it could increase the risk of the development of fungicide resistance. Sheath blight resistance to azoxystrobin has been found for the first time in 2011 in southeast Louisiana, USA.
Significance
Sheath blight was first reported in Japan in 1910. Since then, it has been reported in almost all rice growing areas of the world. The disease has become one of the most economically important diseases of rice throughout the world. R. solani has been classified into 14 anastomosis groups (AGs) based on hyphal interactions. Within a single AG group, intraspecific groups are defined on the basis of morphology, pathology, pectinase isoenzymes, and DNA sequencing. The sheath blight fungus belongs to AG-1 IA. Due to its significance in rice production, extensive research and extension activities have been underway throughout the world with the aim of developing integrated management strategies using resistance varieties, cultural practices, chemical control, and biological control for effective control of sheath blight.
Selected References
Agrios. G. N. 2005. Plant Pathology. 5th edition. Elsevier Academic Press. Ceresini, P. 1999. Rhizoctonia solani. PP-728. Soilborne Plant Pathogens. Don, G., Hollier, C., and Rush, C. Disease management. pp. 82-105. In: Louisiana Rice Production Handbook. Saichuk, J. (ed). LSU Ag Center. Pub. 2321 (3M) 5/14 Rev. Ghosh, S., Kumar, S.K., Jha, G. Identification and functional analysis of AG1-IA specific genes of Rhizoctonia solani. Curr. Genet. 60: 327-341. Kumar, K. V. K., Reddy, M. S., Kloepper, J. W., Lawrence, K. S., Groth, D. E. and Miller, M. E. 2009. Sheath blight disease of rice (Oryza sativa L.) - An overview. Biosci. Biotech. Res. Asia. 6: 465-480. Lee, F. N. and Rush, M.C. 1983. Rice sheath blight: A major rice disease. Plant Dis. 67: 829-832. Uppala, S. and Zhou, X.G. 2018. Field efficacy of fungicides for management of sheath blight and narrow brown leaf spot of rice. Crop Prot. 104: 72-77. Wrather, A. and Sweets, L. 2009. Rice Sheath Blight Control. MP 646. University of Missouri Extension. Yellareddygari, S. K. R., Reddy, M.S., Kloepper, J. W., Lawrence, K. S, Fadamiro, H. 2014. Rice sheath blight: A review of disease and pathogen management approaches. J. Plant Pathol Microb. 5: 241. Zhou, X. G., and Jo, Y. K. 2014. Disease management. pp. 44-57. In: 2014 Texas Rice Production Guidelines. M. O. Way, G. M. McCauley, X. G. Zhou, L. T. Wilson and M. Brandy. (eds.) Texas Rice Research Foundation.
