Revised 2011
Root-knot nematode
Meloidogyne species (~100 species)
Hundreds of species, including fruits, grasses, vegetables, and numerous weeds.

Northern root-knot nematode damage to a carrot
field in New York State. |
Symptoms and signs
Plant Roots
Root-knot nematode symptoms on plant roots are dramatic. As a result of nematode feeding, large galls or "knots" can form throughout the root system of infected plants. Severe infections result in reduced yields on numerous crops and can also affect consumer acceptance of many plants, including vegetables (Figures 1, 2). The degree of root galling generally depends on three factors: nematode population density, Meloidogyne species and "race," and host plant species and even cultivar. As the density of nematodes increases in a particular field, the number of galls per plant also will increase. Large numbers of nematodes penetrating roots in close proximity also will result in larger galls. Meloidogyne hapla (the northern root-knot nematode) produces galls less than half the size of those produced by M. incognita (the southern root-knot nematode) on the same plant hosts. Finally, each crop responds differently to root-knot nematode infection (Figures 3, 4, 5). Carrots typically undergo severe forking with galling predominantly found on lateral roots (Figures 2, 6). Root-knot nematode galls on lettuce are beadlike (Figures 4, 7). On grasses and onions, galls are usually small and barely noticeable, often no more than slight swellings (Figure 8). Depending upon the crop affected and the severity of infection, these symptoms can often result in significant economic losses to growers.

Figure 1 |

Figure 2 |

Figure 3 |

Figure 4 |

Figure 5 |

Figure 6 |

Figure 7 |
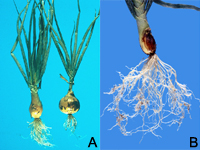
Figure 8 |
Most root-knot nematodes have a very wide host range. Thus, growers who have a root-knot nematode problem may find it difficult to control the nematode and its damage through crop rotation, although this is sometimes a viable option. Cotton growers who have an infestation of M. incognita can often plant peanuts in subsequent years to reduce nematode populations. Unfortunately, peanut is an excellent host for a race of M. arenaria race 1, which can be found in fields that also contain M. incognita. Growers who have a problem with M. javanica can employ sweet pepper as a rotational crop, but not if they also have M. incognita. These two examples demonstrate the importance of understanding which Meloidogyne species is present. In addition to differences in pathogenicity on a specific crop, there can be an even greater degree of specialization. For example, M. hapla will not reproduce on grasses. In contrast, M. graminis only reproduces on grasses. Thus growers who experience problems with M. hapla can rotate corn and wheat into traditional vegetable production, provided they have the appropriate equipment available.
Above Ground Symptoms
While the most diagnostic root-knot nematode damage occurs below ground, numerous symptoms can also be observed above ground. Severely affected plants will often wilt readily. Because galled roots have only limited ability to absorb and transport water and nutrients to the rest of the plant, severely infected plants may wilt even in the presence of sufficient soil moisture, especially during the afternoon. Plants also may exhibit nutrient deficiency symptoms because of their reduced ability to absorb and transport nutrients from the soil. Additional fertilization will not generally result in remediation of root-knot nematode-induced chlorosis. Stunting is frequently observed on host crops grown in root-knot nematode-infested fields, and crop yields are reduced. In highly sensitive crops such as lettuce and carrots, initial density of 2 and < 1 eggs/cc soil, respectively, are sufficient to cause economic losses. At high densities, root-knot nematodes can actually kill host plants, particularly if the high populations occur early in the growing season when plants have minimal root mass (Figure 1).
Above ground symptoms usually appear on clusters of plants (foci). Because nematodes move slowly through the soil, infestations will gradually radiate outward from an initial point of infection. This can result in large foci of affected plants surrounded by seemingly unaffected plants (Figure 1). Cultivation and other practices that physically move soil and plants will rapidly spread root-knot nematodes.
Pathogen Biology
Genus: Meloidogyne
Root-knot nematodes were first reported in 1855 by Berkeley, who observed them causing damage on cucumbers. Until Chitwood's work in 1949, which defined 4 species and one subspecies (M. incognita acrita) within the genus Meloidogyne, the root-knot nematodes were all considered the same species, Heterodera radicola. In an 1887 paper (reprinted in 1892) Goeldi described Meloidogyne exigua, the type species of the genus. From this description, Chitwood obtained the name we currently use for the root-knot nematodes. The name Meloidogyne is of Greek origin, meaning "apple-shaped female." Approximately 100 species of Meloidogyne have been described. The most widespread and economically important species are M. incognita, M. javanica, M. arenaria, M. hapla, M. chitwoodi and M. graminicola. Root-knot nematodes are primarily tropical to sub-tropical organisms, however M. hapla and M. chitwoodi are well adapted to temperate climates.
Like all plant-parasitic nematodes, root-knot nematodes possess a stylet for injecting secretions as well as ingesting nutrients from host plant cells (Figures 9, 10). Nematodes have no internal skeletal framework, and their "skin" or cuticle acts against internal turgor pressure to maintain body shape and aid locomotion.

Figure 9 |
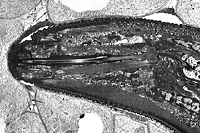
Figure 10 |
Unlike most other plant-parasitic nematodes, root-knot nematode females are globose and sedentary at maturity. They range in length from 400 to 1000 µm. Once they establish a feeding site, they permanently remain at that location within the plant root. The root-knot nematode feeding site is actually a group of cells known as "giant-cells" (Figure 11). When a nematode initially penetrates a plant cell with its stylet, it injects secretory proteins that stimulate changes within the parasitized cells. Parasitized cells rapidly become multinucleate (contain many nuclei) as nuclear division occurs in the absence of cell wall formation. This process is considered to be "uncoupled" from cell division. Cells never actually divide into new cells; they just get bigger and contain more nuclear material. This allows the giant-cell to produce large amounts of proteins which the nematode will then ingest. Giant-cells also act as nutrient sinks, funneling plant nutrients to the feeding nematode. The root-knot nematode does not feed from the cells directly. It forms a feeding tube (from the esophageal gland cell secretions), secreted from the stylet into the plant cell cytoplasm, which acts as a sieve to filter the cytosol that the nematode ingests. As the name implies, giant-cells can grow very large in size. Triggered by nematode esophageal gland cell secretions, an increase in the production of plant growth regulators has been demonstrated to play a role in this increase in cell size and division. Root cells neighboring the giant-cells also enlarge and divide rapidly, presumably as a result of plant growth regulator diffusion, resulting in gall formation.
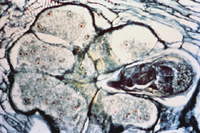
Figure 11 |
As the female nematode enlarges, its posterior region may break the epidermis of the root, and the eggs are deposited into a gelatinous egg mass (Figures 12, 13 14, 15, 16). Mature root-knot females (pearly white in color) can be observed without magnification. Second-stage juveniles (J2) and males can only be observed with the aid of a microscope (Figures 17, 18). Generally, females of root-knot nematodes have a globose body, with a short "neck," containing their stylet, metacorpus and esophageal gland cells.
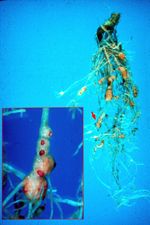
Figure 12 |
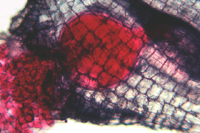
Figure 13 |
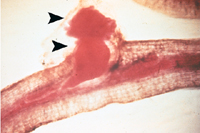
Figure 14 |
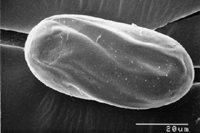
Figure 15 |
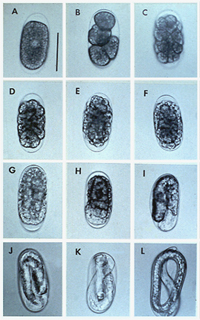
Figure 16 |

Figure 17 |
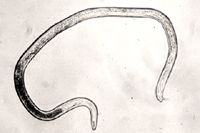
Figure 18 |
The J2s of the root-knot nematode are most commonly encountered in soils and are vermiform (worm-shaped) (Figure 17). They are usually no larger than 500 µm in length and 15 µm in width. This is the only infective stage.
Root-knot nematode males also are vermiform and range from 1100 to 2000 µm in length (Figure 18). They have distinct lips and strongly developed stylets. In addition, they often have visible spicules, for mating, and a blunt, rounded tail. Many Meloidogyne species are parthenogenic or facultatively parthenogenic. This means that males are not necessary to complete the nematode life cycle and viable eggs can be produced by female nematodes in the absence of fertilization. Because of this, males can be rare in a number of species and are only encountered when the nematode population is subjected to an environmental stress.
Root-knot nematodes can be identified to species using a number of techniques, but one common method is perineal pattern analysis (Figure 19). The perineum (the region surrounding the vulva and anus) of female nematodes displays a pattern of ridges and annulations for each species. While some variation does exist among individuals, these patterns are quite consistent within a species. The analysis of isoenzyme electrophoretic profiles, often using esterase and malate dehydrogenase, is a common method for the diagnosis of Meloidogyne species in properly equipped labs. Likewise, DNA analyses can also be used to identify different species of root-knot nematodes.
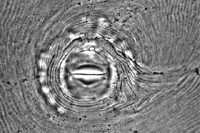
Figure 19 |
Disease Cycle and Epidemiology
Epidemiology
Root-knot nematodes begin their lives as eggs that rapidly develop into J1 (first-stage juvenile) nematodes (Figure 16). The J1 stage resides entirely inside the translucent egg case, where it molts into a J2 nematode. The motile J2 stage is the only stage that can initiate infections (Figure 20). J2s attack growing root tips and enter roots intercellularly, behind the root cap (Figure 21). They move to the area of cell elongation where they initiate a feeding site by injecting esophageal gland secretions into root cells. These nematode secretions cause dramatic physiological changes in the parasitized cells, transforming them into giant-cells (Figure 11). If the nematode dies, so will the giant-cells upon which it feeds.

Figure 16 |
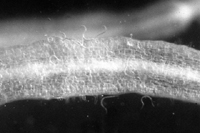
Figure 20 |
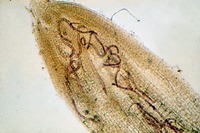
Figure 21 |

Figure 11 |
J2s do not possess reproductive organs. As with all nematodes, root-knot nematodes undergo four juvenile stages, each progressing through a "molting" process similar to that of insects. As a result of this process, juvenile root-knot nematodes have little resemblance to adult males and females. In the J4 stage, the progression from juvenile to globose adult females or to vermiform adult males becomes clearly visible. They emerge as adults from the J4 cuticle (Figure 22). A single female nematode can produce 500 to more than 1000 eggs (Figures 13, 23).
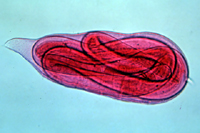
Figure 22 |

Figure 13 |

Figure 23 |
The length of a root-knot nematode life cycle varies among species but can be as short as two weeks. Nematodes in cooler regions typically have longer life cycles. Eggs may remain inside root tissue or may be released into the soil matrix. Eggs hatch at random, i.e. hatching does not require exposure to root exudates. Under favorable conditions, root-knot nematode eggs have been reported to survive for at least one year in the soil.
Disease Management
Cultural Controls
The use of cultural control methods to manage root-knot nematodes is the most environmentally sustainable and potentially most successful method for limiting root-knot nematode damage. However, because root-knot nematodes have very large host ranges, cultural control methods require careful planning. Vegetable fields infested with M. hapla can potentially be planted to a nonhost crop such as corn, but the grower's short-term economic return could be diminished. In addition, the grower may have to invest in new equipment or subcontract for some labor. If the grower can identify an alternative nonhost crop with high economic return, crop rotation can be very successful. In contrast, M. incognita on cotton, as discussed earlier (see Symptoms), can usually be managed effectively with crop rotation
Another cultural control strategy is the use of cover crops. Cover crops can be grown outside of the normal agricultural growing season, and some are antagonistic to nematodes. Cover crops such as sudangrass and marigolds actually produce chemicals that are toxic to nematodes. Cover crops have the added benefits of stabilizing topsoil and improving soil quality. As with crop rotation, however, specialized equipment may be required to deal with different cover crops. Other techniques, including flooding and solarization of fields, have controlled nematodes, but only in warm climates and when a particular field can be removed from cultivation for long periods during the treatment. While cultural control methods are extremely valuable tools, they require extensive consideration, planning and economic investment before successful implementation can be achieved.
Chemical Control
Root-knot nematodes are very difficult to manage because they are soilborne pathogens with a wide host range. Because root-knot nematodes live in the soil, chemical control requires applications of large amounts of chemicals with specialized equipment. Fumigants (such as 1,3-dichloropropene, methyl bromide and dazomet) are commonly applied as pre-plant treatments to reduce nematode numbers, but they must thoroughly penetrate large soil volumes to be effective. Some fumigants volatilize very quickly, so treated soil must have a cover or tarp to maintain the fumigant in the soil long enough to be effective. The phasing out of methyl bromide (an efficient fumigant) has intensified the search for alternatives that can be used by farmers.
In addition to broad-spectrum fumigants, nervous system toxins (including oxamyl and fenamiphos) have been shown to be extremely effective for controlling root-knot nematodes (Figure 24). Because both nematodes and humans have nervous systems, chemical treatments that target nematode nerves also are a potential danger to human nerves. These chemicals (carbamates and organophosphates) are extremely toxic to humans and other nontarget organisms. Currently, these chemicals are the most economically feasible control method for root-knot nematodes. Because they are not toxic to plants, they are the only chemical options for established plants.

Figure 24 |
Plant Resistance
In certain crops, root-knot nematodes are effectively controlled by resistance genes (Figure 4). In tomato, genetic resistance to root-knot nematodes is conferred by the Mi gene which was obtained from Lycopersicon peruvianum, a wild relative of the common tomato. When resistance genes are transferred into susceptible germplasm, the genetically altered plants become resistant to infection by certain species of root-knot nematode. However, populations of root-knot nematodes that can circumvent root-knot resistance have been identified in both the greenhouse and agricultural fields, suggesting the potential for the eventual failure of root-knot resistance. Many other resistance genes have also been identified that are effective against species of Meloidogyne. These include the Mi2 through Mi8 genes (all from Lycopersicon) and the Me and N genes from pepper. In many cases, however, these genes become ineffective at higher temperatures. Besides these genes, there are a number of genes that have not yet been named, and new sources of genetic resistance to root-knot nematodes are frequently being identified.

Figure 4 |
|
Biological Control
Control measures employing organisms antagonistic to root-knot nematodes have been attempted by many researchers. The most commonly used biological control agents are fungi and bacteria. There are many kinds of nematophagous (nematode-feeding) fungi. Some fungi use mycelial traps or sticky spores to capture nematodes (Figures 25, 26), for example, Arthrobotrys spp. and Monacrosporium spp. Other fungi parasitize eggs and root-knot nematode females, e.g., Pochonia chlamydosporia and Paecilomyces lilacinus. The major bacterial antagonists are Pasteuria penetrans and species of Bacillus. Endospores of P. penetrans attach to the cuticle of a juvenile nematode, produce penetration structures that enter the nematode, and slowly consume it. Several nematode antagonists have been studied in both greenhouse and field experiments. A number of commercial products based on biocontrol agents are available for the management of root-knot and other nematodes. However, a significant problem in developing effective biological control agents is the inability to economically generate the large amounts of biological material necessary for application over large areas.

Figure 25 |
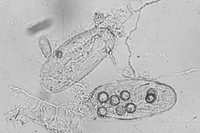
Figure 26 |
Integrated Management
The most successful approaches to nematode control rely on integrated pest management strategies (IPM). IPM combines management options to maintain nematode densities below economic threshold levels. IPM techniques can still be difficult to implement against a pathogen as aggressive and resilient as root-knot nematodes. Nevertheless, a combination of management tactics/tools, including cultural practices (rotations with nonhost crops and cover crops that favor the build-up of nematode antagonists), resistant cultivars, and chemical soil treatments, if necessary, generally provide acceptable control of root-knot nematodes. The extent of this success, however, is dependent upon having accurate damage threshold densities and available and readily acceptable resistant cultivars.
Significance
Nematodes account for an estimated 14% of all worldwide plant losses, which translates into almost $100 billion dollars annually. By far, root-knot nematodes are the most common and destructive nematode pathogens. They produce some of the most dramatic symptoms and can substantially reduce crop yields (Figure 1). Root-knot nematodes are found in all agricultural regions worldwide. They can survive in temperate climates and can devastate crops grown in the tropics (Figures 27, 28). Most root-knot nematodes also have extremely wide host ranges. Although it is difficult to ascertain the number of hosts for any one root-knot nematode species, it is likely that some root-knot nematodes can survive on hundreds of different plant species. This can make it extremely difficult to control a root-knot nematode problem, particularly if the nematode can survive on weeds. In addition, root-knot nematodes have repeatedly been shown to predispose their host plants to infection by other crop pathogens, increasing the potential for crop loss.

Figure 1 |
|

Figure 27 |
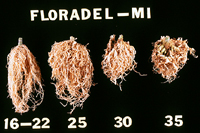
Figure 28 |
Selected References
Barker, K.R., G.A. Pederson and G.L. Windham. 1998. Plant and Nematode Interactions. ASA, CSSA, SSA Publishers, Madison, WI.
France, R.A. and G.S. Abawi. 1993. Interaction between Meloidogyne incognita and Fusarium oxysporum f. sp. phaseoli on selected bean genotypes. Journal of Nematology 26:467-474.
Jepson, S.B. 1987. Identification of Root-Knot Nematodes (Meloidogyne Species). CAB International, Wallingford, UK.
Karssen, G. 2002. The Plant-Parasitic Nematode Genus Meloidogyne Goeldi, 1892 (Tylenchida) in Europe. Brill Academic Publishers, Boston, MA.
Lamberti, F. and C.E. Taylor, Eds. 1979. Root-Knot Nematodes (Meloidogyne Species). Academic Press, New York.
Perry, R.N., M. Moens and F.J. Starr, Eds. 2009. Root-Knot Nematodes. CAB International, Wallingford, UK.
Sasser, J.N. and C.C. Carter, eds. An Advanced Treatise on Meloidogyne: Volume I, Biology and Control. 1985. Department of Plant Pathology and Genetics, North Carolina State University and the United States Agency for International Development, Raleigh, NC.
Starr J.L, J. Bridge and R. Cook, eds. Plant Resistance to Parasitic Nematodes. 2002. CABI Publishing, Cambridge, MA.
Viaene, N.M. 1998. Management of Meloidogyne hapla on lettuce in organic soil with sudangrass as a cover crop. Plant Disease 82:945-952.
