Soil-borne wheat mosaic
Soil-borne wheat mosaic virus
Wheat, triticale, rye, barley, and other grasses

Close-up of SBWM nursery
Symptoms and Signs
Soil-borne wheat mosaic (SBWM) affects autumn-sown small grains. The first visual evidence of
Soil-borne wheat mosaic virus (SBWMV) infection in a growing season usually occurs in the spring, after the crop begins to green up. However, in some years SBWM symptoms are expressed in the late autumn or early winter, especially in warmer climates. Irregular, chlorotic patches in the field suggest soil-borne viral infection, although similar symptoms may be caused by a number of other viruses or other biotic or abiotic factors. In dry environments, patches of symptomatic SBWMV-infected plants usually occur in low-lying, wet regions of the field (Figure 2) that are conducive for infection by the swimming zoospores of its protozoan viral vector,
Polymyxa graminis. In wetter or more humid climates, these patches may occur anywhere in the field (Figure 3).

Figure 2 |

Figure 3 |
Upon closer inspection, SBWMV-infected plants are often stunted (Figure 4) and the leaves may have a general chlorotic mosaic or irregular mottling and streaking (Figure 5). A few strains of the virus cause rosetting in highly susceptible wheat cultivars; leaves and tillers remain short, growth is bunchy or compact, and tillering is excessive (Figure 6). Perhaps the most diagnostic characteristic of SBWMV infection is that symptoms are not expressed on leaves that emerge after the average temperature rises above 20 °C (68 °F). Thus, SBWM chlorotic patches tend to disappear in late spring. SBWM is most often confused with a disease caused by another soil-borne virus,
Wheat spindle streak mosaic virus (WSSMV), which can also induce stunting and chlorosis and is also temperature sensitive. A good diagnostic feature to distinguish between these two viruses is that WSSMV induces chlorotic streaks that are elongated and spindle-shaped and often have a dark green island in their center (Figure 7).

Figure 4 |

Figure 5 |

Figure 6 |
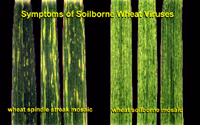
Figure 7 |
However, accurate diagnosis of SBWM is complicated by the fact that symptom expression varies by host genotype and, as previously mentioned, similar symptoms may be caused by a range of biotic and abiotic factors. Therefore, additional diagnostic tests are often conducted to verify the presence of SBWMV. The most commonly used tests are detection of viral coat protein by enzyme-linked immunosorbent assay (ELISA) or detection of a portion of the RNA genome by reverse-transcription polymerase chain reaction (RT-PCR).
Pathogen Biology
SBWMV is the type member of the
Furovirus genus. Members of the genus are characterized by rigid rod-shaped particles (Figure 8) and positive sense (directly serving as mRNA) RNA genomes consisting of two molecules that are packaged into separate particles (Figure 8). The two particles differ in length, and the shorter particle (138-160 nm) is 10 to 20 times more prevalent in infected tissue than the longer particle (281-300 nm), but both particles are required for infection.
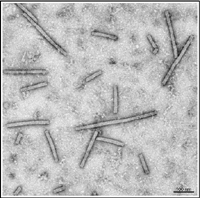
Figure 8 |
The longer particle contains RNA 1, which is approximately 7100 nucleotides long and encodes three proteins. Two of these, measuring 150 kDa and 209 kDa, are associated with virus replication (Figure 9). The 37 kDa protein is a cell-to-cell movement protein. The 150 kDa and 209 kDa proteins are translated directly from the message sense viral RNA, whereas the 37 kDa protein is expressed via a subgenomic mRNA.
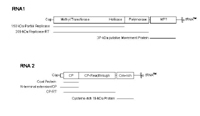
Figure 9 |
The shorter particle contains RNA 2 (approximately 3600 nucleotides), which also encodes three different proteins (Figure 9). The first is the 19 kDa coat or capsid protein (CP). Sometimes, the coat protein UGA termination codon is suppressed allowing translation of an 84 kDa CP-readthrough protein, which is thought to be required for virus transmission by its vector,
Polymyxa graminis (Figure 10). The third protein is a 19 kDa cysteine-rich protein that is expressed via a subgenomic mRNA and may function as a suppressor of post transcriptional gene silencing (Figure 9). Post transcriptional gene silencing is a mechanism that can provide host resistance to a virus.

Figure 10 |
Systemic infection of plants is temperature dependent. Virus movement from the roots (site of infection) to the leaves occurs at temperatures less than 20 °C (68 °F). Higher temperatures limit virus movement into the leaves and if the temperatures rise after some leaves are infected, no further infection of developing leaves will occur. If SBWMV-infected plants are grown at high temperatures (25-30 °C/ 77-86 °F), or for prolonged periods of time, or if the virus is maintained by repeated mechanical inoculation, the virus tends to delete specific sequences in the gene encoding the CP-readthrough protein (Figure 11). There is a tendency for virus strains that contain large deletions to be more virulent when mechanically inoculated onto wheat plants. Stable deletion mutants of one particular size can predominate, but this tends to be cultivar specific. A 759 nucleotide deletion predominated in the virus maintained in the cultivar ‘Galahad’ after repeated mechanical inoculations, while a stable deletion of 1058 nucleotides predominated in the virus maintained in the cultivar ‘Michigan Amber.’ Since these deletions are generated and maintained in the absence of transmission by the vector, the CP readthrough domain has been implicated in vector transmission.
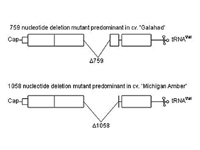
Figure 11 |
Disease Cycle and Epidemiology
Polymyxa graminis (Figure 10), the vector of SBWMV, belongs to the endoparasitic slime molds (Plasmodiophoromycota). This organism produces resting spores that harbor viral RNA and movement protein. These resting spores can remain dormant in soil for up to 30 years, waiting to infect the next graminaceous host germinating in an environment conducive for infection, at which time the virus-containing zoospores germinate. Soil water is critical for the swimming zoospore to reach a host root. The threshold matric potential is between –20 and –40 kPa. This means that soil pores between 7.4 and 14.7 μm must be filled with water in order for significant transmission to occur.
On reaching the host root, a
P. graminis zoospore encysts on the surface of a cortical root cell, and develops a spear-like "stachel," which when mature will shoot through the adjoining zoospore and host walls (Figure 12). Along with the stachel, the zoospore contents and presumably the contaminating SBWMV particles are emptied into the host cortical cell. Thus begin the life cycles of the vector and of the virus. How the virus is attached to or carried by the zoospore, and exactly how the virus is transferred from the zoospore to the plant root are not fully understood, although SBWMV RNA and movement protein, but not capsid protein, have been detected within
P. graminis sporosori.
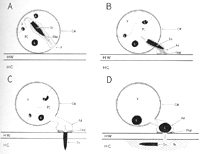
Figure 12 |
Optimal temperatures for
P. graminis infection vary among virus isolates and often reflect temperatures where the isolate is commonly found. Thus, isolates from India are active at warmer temperatures (27-30 °C/80-86 °F) than isolates from Belgium, Canada, Japan and France (15-18 °C/ 59-64 °F). In New York state, the optimal temperature for transmission of SBWMV is 15 °C (59 °F), with significant transmission occurring in the growth chamber in less than 24 hours, given conducive soil moisture. Importantly, no significant transmission occurs at 6.5 °C (44 °F), suggesting that SBWMV is either transmitted in the autumn or in the spring in temperate climates, but not during the winter months. Given the period of time required for SBWMV systemic movement and symptom expression and the brevity of spring temperatures conducive to SBWM symptom expression, spring infections by
P. graminis are not likely to contribute to viral symptom expression.
After the stachel-mediated infection, one of two types of plasmodia (multinucleate non-motile masses of protoplasm) of
P. graminis may form in a single host cortical root cell (Figure 13). The plasmodia differentiate to give rise either to secondary zoospores or resting spores.
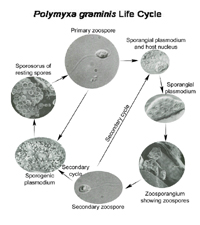
Figure 13 |
Infection of plant roots by
P. graminis is the first step in the poorly defined SBWMV infection process. Viral replication within the initially infected cell and movement to adjacent host cortical cells does not appear to be inhibited in wheat genotypes resistant to systemic foliar SBWMV infection. There is some evidence that SBWMV moves systemically via xylem. Since viruses move within the living cell contents of plant tissues, this suggests that SBWMV must be inoculated into young tissues before the living protoxylem cells have differentiated to become mature, dead xylem cells. Protoxylem infection is most likely to occur at root tips and where lateral roots initiate, which coincides with the region of the rhizosphere that is most likely to attract
P. graminis zoospores.
Disease Management
Resistance to the virus
The most practical disease control strategy against SBWM is cultivar resistance, first described by H.H. McKinney in 1925 (Figure 14). The mechanism of cultivar resistance has yet to be fully characterized, although preliminary evidence suggests that resistance prevents systemic movement from roots to foliage, but does not prevent replication and cell-to-cell movement in roots. At temperatures above 20 °C (68 °F), resistance to foliar infection breaks down, but symptoms do not result. McKinney rightly noted that even genotypes with field resistance were susceptible to mechanical foliar inoculations. Resistance is conferred by 1 to 3 genes. Given the strong environmental influence on transmission and symptom expression, the broad sense heritability estimate of 43-55% reflects the high heritability of resistance. This means that about 50% of the variation in the number of symptomatic plants is due to genetically inherited host resistance.

Figure 14 |
Resistance to the vector
Several studies in barley suggest that all cultivars tested are susceptible to
P. graminis infection, but also reveal a cultivar-dependent, quantitative variation in the number of zoospores released. Furthermore, isolates of
P. graminis show host species specificity, which may limit the ability of
P. graminis isolates to transmit the virus.
Varietal mixtures
Based on what is known about the SBWMV pathosystem, mixtures of resistant and susceptible host genotypes could theoretically provide protection against pathogens through reduced density of susceptible genotypes and barrier effects (Figure 15). However, because secondary zoospores are limited in their dispersal distance, it is more likely that a secondary zoospore would infect the plant from which it is derived (autoinfection) than another plant (alloinfection). Typically, there would be a maximum of one generation of secondary zoospores in the autumn, when the most damaging SBWMV transmission occurs. Therefore, one would not expect to observe significant benefits from varietal mixtures. However, Hariri et al. (2001) reported intriguing results that 1:1 and 1:3 (susceptible:resistant) mixtures developed significantly less disease than predicted by pure stand comparison.
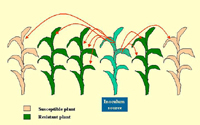
Figure 15 |
Chemical control
The only chemicals that have been reported to provide reproducible control against
P. graminis in the field are soil fumigants, which are not economically feasible in small grains systems.
Crop rotation
Continuous wheat amplifies
P. graminis inoculum, and SBWM-susceptible cultivars increase the proportion of viruliferous zoospores. However, because of the long-term survival of the virus in
P. graminis resting spores, crop rotation is not an effective management strategy for SBWM.
Sanitation
Any equipment or mechanism that transports soil has the potential to transport SBWMV-infested inoculum. In growers’ fields in New York State, where SBWMV was recently introduced, we observe SBWMV-infected patches most often associated with areas where machinery first entered the field. This observation stresses the importance of sanitation (e.g., cleaning machinery) to avoid the introduction of new soil-borne viruses (Figure 16).

Figure 16 |
Historical Significance
Early history of SBWMV research
Harold H. McKinney first described soil-borne wheat mosaic, which he initially called “rosette,” in Illinois in 1919 and through a series of experiments concluded that the causal agent of “rosette” was a virus (Figure 17). Soon McKinney realized that the virus was soil-borne; that some cultivars expressed the rosette symptoms, some the mosaic, and some were resistant to both symptoms; and that, although the disease occurred predominantly in winter wheat and winter rye, during prolonged cool weather in the spring even spring grains could show mild symptoms. In 1925, SBWM was identified at the Arlington Experiment Farm in Virginia, and in the following year McKinney was transferred to the Arlington farm to lead research on viral diseases of cereals. The land that was then Arlington Experiment Farm (Figure 18) is currently the site of the Pentagon.

Figure 17 |

Figure 18 |
Early history of
Polymyxa graminis research
Polymyxa graminis was first discovered in 1929 and was thoroughly described by G. A. Ledingham in 1939 as part of a doctoral thesis submitted to the University of Toronto. M. K. Brakke and co-workers first correlated vector (P.
graminis) and viral (SBWMV) infections in the 1960s, and provided some evidence that SBWMV is internalized by its vector.
Economic significance
Early in the history of SBWM research, host genotypes susceptible to rosette stunting (Figure 15) were common, and the resulting yield loss was potentially greater than 50%. As a result of the exclusion of cultivars exhibiting rosette symptoms, the devastating rosette phenotype is seldom seen today. However, many contemporary cultivars still exhibit the mosaic phenotype and may experience significant yield losses. In many grain producing regions, SBWM results in significant annual yield losses, but, since SBWM symptoms are short-lived and mimic nutritional deficiencies, its economic significance is often underestimated. SBWMV infection causes a reduction in kernel weight, tiller number, and test weight, cumulatively leading to lower grain yields.
World-wide distribution
SBWMV is currently distributed throughout most of the eastern and central U.S. Since the first European report in 1960, SBWMV has spread rapidly on the continent and is now widely distributed in France, Germany, and Italy. In the U.K., SBWMV was first found affecting winter wheat crops on a farm in Wiltshire in 1999, and subsequently on two farms in Kent in 2000. SBWMV is also found in Argentina and Brazil. The Japanese isolate of SBWMV differs significantly from the type isolate, but is still considered to be the same virus.
Selected References
Barbosa, M. M., L.R. Goulart, A. M. Prestes, and F. C. Juliatti. 2001. Genetic control of resistance to
Soil-borne wheat mosaic virus in Brazilian cultivars of
Triticum aestivum L.
Thell. Euphytica 122:417-422.
Cadle-Davidson, L., Schindelbeck, R. R., van Es, H. M., Gray, S. M., Bergstrom, G. C. 2003. Using air pressure cells to evaluate the effect of soil environment on the transmission of soilborne viruses of wheat. Phytopathology 93:1131-1136.
Driskel, B. A., P. Doss, L. J. Littlefield, N. R. Walker, J. J. Verchot-Lubicz. 2004.
Soil-borne wheat mosaic virus movement protein and RNA and
Wheat spindle streak mosaic virus coat protein accumulate inside resting spores of their vector,
Polymyxa graminis. Molecular Plant-Microbe Interactions 17:739-748.
Hariri, D., M. Fouchard, and H. Prud'homme. 2001. Incidence of
Soil-borne wheat mosaic virus in mixtures of susceptible and resistant wheat cultivars. European Journal of Plant Pathology 107:625-631.
Littlefield, L. J., J. H. Whallon, P. J. Doss, and Z. H. Hassan. 1998. Postinfection development of
Polymyxa graminis in roots of
Triticum aestivum. Mycologia 90:869-882.
Shirako, Y., and T. M. A. Wilson. 1993. Complete nucleotide-sequence and organization of the bipartite RNA genome of
Soil-borne wheat mosaic virus. Virology 195:16-32.
Verchot, J., B. A. Driskel, Y. Zhu, R. M. Hunger, and L. J. Littlefield. 2001. Evidence that
Soil-borne wheat mosaic virus moves long distance through the xylem in wheat. Protoplasma 218:57-66.
Yamamiya, A., and Y. Shirako. 2000. Construction of full-length cDNA clones to
Soil-borne wheat mosaic virus RNA1 and RNA2, from which infectious RNAs are transcribed
in vitro: Virion formation and systemic infection without expression of the N-terminal and C-terminal extensions to the capsid protein. Virology 277:66-75.
