Updated 2021.
Soybean cyst nematode disease
Heterodera glycines
Soybean (primary economic host), other legumes
Authors
Eric L. Davis, North Carolina State University, Raleigh
Gregory L. Tylka, Iowa State University, Ames

Soybean field with symptoms of soybean cyst nematode damage
Microscopic worms burrowing through soybean roots. Injecting invisible foreign substances into plant cells using mouthparts shaped like hypodermic needles. Forcing living plant cells to develop into grotesquely large, hyperactive food factories. Feeding as they shapeshift and swell. Eventually erupting from the roots as bloated females engorged with hundreds of eggs. The eggs, lying dormant in soil for up to 10 years within their mother's hardened body. Waiting to infect again. Does this sound like the plot of a science fiction movie? Believe it or not, this is the basic biology of a widespread, serious pathogen of soybeans - the soybean cyst nematode (SCN).
Symptoms and Signs
High numbers (population densities) of the soybean cyst nematode (SCN) can result in large portions of soybean fields with plants that are severely stunted and yellow or dead (Figures 1 and 2). More frequently, however, few aboveground symptoms of SCN will be seen (Figure 3) - yet soybean yield losses of 10-30% or more can be attributed to SCN damage in these fields. This “hidden" nature of SCN yield loss presents a major problem for soybean growers who must learn to recognize fields that are infested with the nematode. Also, symptoms and reduced yields in SCN-infested fields often are mistakenly attributed to adverse cultural or environmental conditions rather than to SCN - a tragic mistake that leads to increasing SCN numbers and greater damage and yield losses in subsequent years. The age and vigor of the soybean crop, the nematode population density in the soil, levels of soil fertility and moisture, and other environmental conditions influence the appearance and intensity of symptoms. Soybean cyst nematode damage usually is more severe in light, sandy soils and in dry growing seasons, but symptoms and damage can occur in all types of soil and weather.

Figure 1. Severe stunting and yellowing caused by SCN.
|

Figure 2. Aerial view of widespread, severe SCN damage in a field.
| 
Figure 3. SCN-infested field with few obvious symptoms.
|
The whitish-yellow adult females of SCN that are visible on the outside of roots with the unaided eye are a telltale sign of SCN infestation (Figure 4). The SCN females are slightly less than 1 mm (1/25 inch) in diameter and considerably smaller than nitrogen-fixing
Bradyrhizobium nodules that occur naturally on healthy roots (see arrows in Figure 5). Reduced nodulation has been associated with infection of soybean roots by some populations of SCN, but it is not a consistent and obvious symptom of SCN. Other than observing white SCN females on roots, the only other reliable means to confirm an SCN infestation in a field is through analysis of soil and root samples by diagnostic laboratories (see section titled “Scout for early detection" under “Disease Management" below). The vermiform (worm-shaped) juveniles and adult nematode males can be extracted from soil samples, as can the brown cysts (dead female body containing eggs) (Figure 6). Developing SCN juveniles and females can be seen in stained soybean root samples (Figure 7). Diagnostic labs can estimate the population density of SCN in a field based on soil sample results and provide recommendations of SCN management options.

Figure 4. White SCN females on soybean root.
| 
Figure 5. White SCN females (yellow arrows) on soybean root with nitrogen-fixing nodules (red arrows).
|
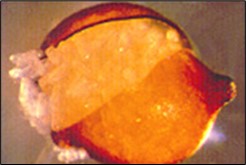
Figure 6. SCN cyst (dead female) full of eggs recovered from soil.
| 
Figure 7. SCN juveniles (stained purple) within soybean root. |
Pathogen Biology
There are numerous different species of cyst nematodes that feed on plants. Early reports of cyst-forming nematodes on soybeans from Asia classified them as variants of the sugar beet cyst nematode,
Heterodera schachtii, and occasionally as the pea cyst nematode,
Heterodera goettingiana. Japanese nematologist M. Ichinohe made SCN a distinct species,
H. glycines, in 1952. Morphological features of the adults of SCN are used for species identification. SCN adults are sexually dimorphic, meaning that females and males have different body shapes. The females are swollen and sedentary (immobile), and the males are vermiform (worm-shaped) and motile. Only the swollen female stage of SCN on the surface of soybean roots can be seen with the unaided eye (Figures 4 and 5). Males and juvenile stages of SCN must be extracted from soil or plant roots and viewed under a microscope.
The second-stage juveniles (J2) of SCN, which hatch out of the eggs, are worm-shaped, 375-520 µm (1/50 to 1/68 inch) long, and about 18 µm (1/1,500 inch) in diameter. The overall body shape of the nematode is maintained by the pressure of its internal body fluids pushing against its strong, but flexible, outer body wall or "cuticle" (like a water balloon). The outside of the cuticle has a series of fine rings (annulations), like an accordion, that allows the cuticle to bend at any point along the nematode body. The cuticle is composed mainly of the structural protein collagen, and the cuticle is shed (molted) four times to allow growth and maturation of the nematode. There is no chitin in the body of the nematode except for a thin layer in the shell of eggs. The "head" or anterior region of the nematode can be recognized by the presence of a short, dark spear with basal knobs (the "stylet") positioned just inside the tip of the head (Figure 8). The stylet is hollow (like a hypodermic needle) and protrudes from the head when used by the nematode for penetrating plant tissues and feeding from plant cells. The very outer tip of the nematode head (called the "lip" region) is slightly elevated, rounded, and darkened in J2 of SCN. A round, muscular pumping organ called the metacorpus can be seen in the relatively clear area just behind the stylet called the esophagus. The metacorpus pumps substances (i.e., nematode secretions and food) in and out of the esophagus of the nematode. Just below the metacorpus is another relatively translucent area that contains three esophageal glands that overlap the nematode's intestine on the ventral (stomach) side of its body. The intestine can be recognized as a fairly long, dark area extending from the esophageal glands to the tail of the nematode. The tail of SCN J2 tapers uniformly to a fine, rounded tip that is hyaline (clear).
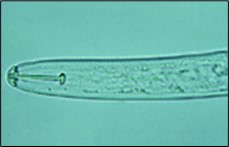
Figure 8. Head (anterior) region of SCN second-stage juvenile with stylet.
| 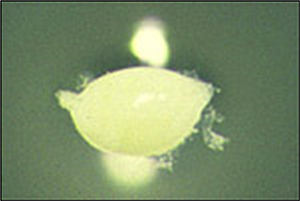
Figure 9. Lemon-shaped adult SCN female.
| 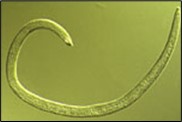
Figure 10. Adult SCN male.
| 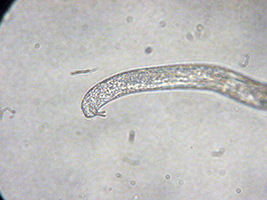
Figure 11. Tail of SCN adult male with spicules.
|
Adult females of SCN are the most easily identified stage. The females are from 500 to 900 µm (1/30 to 1/50 inch) long and 200 to 700 µm (1/36 to 1/125 inch) wide (Figure 9). The vulva and anus collectively form a cone-shaped projection from the rear of the female's body called the vulval cone, resulting in the characteristic overall "lemon" shape of the female's body. The SCN females swell and molt while developing within the root through juvenile life stages until they erupt from the plant roots (see Disease Cycle). The adult females of SCN initially are white (on the root surface), and their heads are buried within the root and attached to the plant vascular tissue for feeding. As the female ages, the body turns yellow and eventually brown as she dies. The female body eventually becomes hardened and forms a protective cyst encasing the eggs.
Adult males of SCN are worm-shaped and relatively long (Figure 10), compared to second-stage juveniles. They are 1,200 to 1,400 µm (1/20 to 1/25 inch) long and 26 to 30 µm (1/800 to 1/1,000 inch) wide. Although the head, stylet, and esophagus of the adult male are similar to that of the second-stage juveniles, the tail of males is short with a blunt, rounded terminus. An easy, defining characteristic of males is the presence of two dark hooks, called "spicules" that are always present sticking out of the male body near the tail (Figure 11) and are involved in mating.
Disease Cycle and Epidemiology
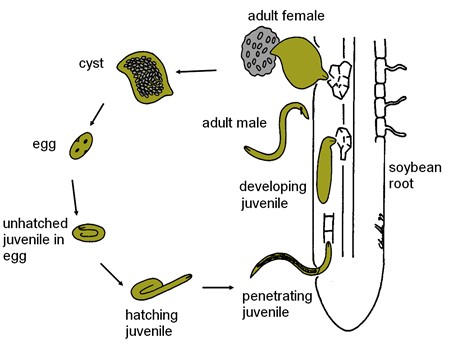
Like all nematodes, SCN has six life stages - egg, four juvenile stages (abbreviated J1 to J4), and the adult stage (female and male). The SCN life cycle takes about three to four weeks to complete under optimum conditions, but this timing may be influenced by environmental conditions (mainly temperature and moisture). In most locations where soybeans are grown, several generations of SCN can be completed in a typical growing season. After an embryo develops within the egg to the first-stage juvenile, the nematode goes through four molts to the adult stage. The first molt occurs in the egg resulting in the formation of the second-stage juvenile (J2) within the eggshell prior to hatching (Figure 12), and it is the J2 that emerges from the egg (Figure 13). Hatching seems to have evolved as a survival strategy in SCN. A low percentage of SCN eggs hatch spontaneously. A significant proportion of eggs that are retained within cysts may not hatch until they sense that soybeans are present in the soil. It is hypothesized that exudates from soybean roots stimulate hatching. Another proportion of eggs within cysts do not hatch even when conditions are favorable - they are in a state called diapause. Diapause appears to be a time-mediated hatching process, the basis of which is not presently understood.
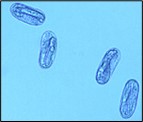
Figure 12. Eggs containing SCN second-stage juveniles.
| 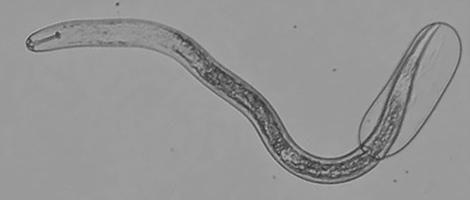
Figure 13. SCN second-stage juvenile hatching from egg (courtesy N. Schroeder, Univ. Illinois).
|
The J2 is the stage of SCN that infects soybean roots. The J2 migrates in soil, penetrates plant roots completely (Figure 14), and moves intracellularly (burrowing through plant cells) to the root vascular tissue, often leaving a zone of visible root necrosis (dead plant cells) along their migratory path within the root. The J2 thrusts its stylet and secretes cell-wall-degrading enzymes (cellulases) to migrate directly through plant cells (Figure 15). When the J2 reaches the root vascular tissue, nematode stylet secretions modify specific plant cells into an elaborate feeding site called a syncytium (Figure 16). The J2 will not develop beyond the J2 stage unless a syncytium is formed for feeding. The syncytium is a large, metabolically active feeding site that becomes multinucleate as neighboring plant cells are incorporated into the syncytium by cell wall dissolution and cell fusion. The J2 feeds from the syncytium and begins to swell and become immobile.
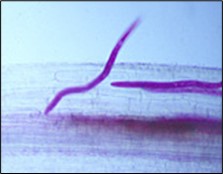
Figure 14. Second-stage SCN juvenile (stained purple) penetrating soybean root.
| 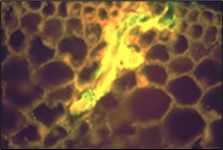
Figure 15. Cell-wall-degrading enzymes (stained yellow) secreted by penetrating SCN juvenile within soybean root.
| 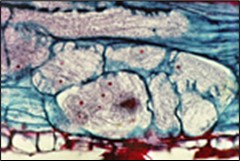
Figure 16. Multinucleate feeding site (syncytium) formed by SCN within soybean root.
|
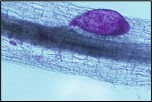
Figure 17. Young SCN female (stained purple) emerging from soybean root.
| 
Figure 18. Mature adult SCN female with egg mass on soybean root.
| 
Figure 19. Newly formed adult SCN male still inside cuticle of swollen fourth-stage juvenile.
|
The subsequent juvenile stages (J3 and J4) molt and continue to enlarge as the nematode feeds. Approximately half of the juveniles will become swollen females, and the majority of the female body (except for the head) breaks through the root surface and becomes visible on the surface of the root (Figures 17, 18). Males develop coiled within the swollen J4 cuticle (Figure 19), and they emerge from the cuticle and root as mobile, vermiform adult nematodes (Figure 10).
Males do not feed, but they mate with females on the root surface through a process called sexual reproduction. After fertilization of females by males, the majority of the 200-500 eggs produced by the female are retained within its body, but some eggs may be laid in a gelatinous matrix extruded from the posterior (vulva) of the female. As the egg-filled female eventually dies, its cuticle becomes a brown, hardened structure called a cyst that encases and protects hundreds of viable eggs within (Figure 6). Cysts dislodge from roots and exist free in the soil.
Epidemiology
As with most plant-parasitic nematodes in soil, soybean cyst nematodes do not move far from the root zone that they currently infest under their own power. Instead, passive movement of SCN within a field occurs by factors that naturally move soil and results in small patches of infested areas gradually enlarging and developing into significant areas of disease. Infested areas become much more pronounced in locations in soybean fields that are under environmental stress (such as insufficient fertilization or water). The spread of SCN within fields can be accelerated by cultural practices of the grower (such as by plowing, disking, or soil cultivation). SCN can be introduced to noninfested fields on farm equipment carrying SCN-infested soil. The cysts of SCN are lightweight and can survive desiccation. They can be moved in surface water, by wind, and also by the movement of animals. It can take from 5-9 years from the time of introduction of SCN into a field until the SCN populations increase to detectable levels. By that time, infestation with SCN has become a permanent problem in that field.
Disease Management
Once established in a field, SCN cannot be eradicated. However, there are various practices that can be used in an active, integrated pest management (IPM) program to minimize the build-up of SCN numbers and maximize soybean yields in infested fields.
Scout fields for presence of SCN
One cannot rely upon aboveground symptoms to indicate that SCN is present in a field because such symptoms of SCN can be absent. Given the widespread distribution of SCN throughout the soybean-growing areas of the United States and Canada (Figure 20), every field in which soybeans are grown should be checked for the presence of SCN.
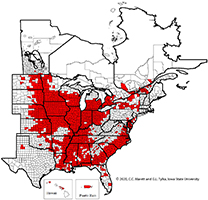
Figure 20. Map of counties and rural municipalities (in Canada) in which SCN was discovered between 1954 and 2020.
| 
Figure 21. Digging soybean roots mid season to look for adult SCN females.
| 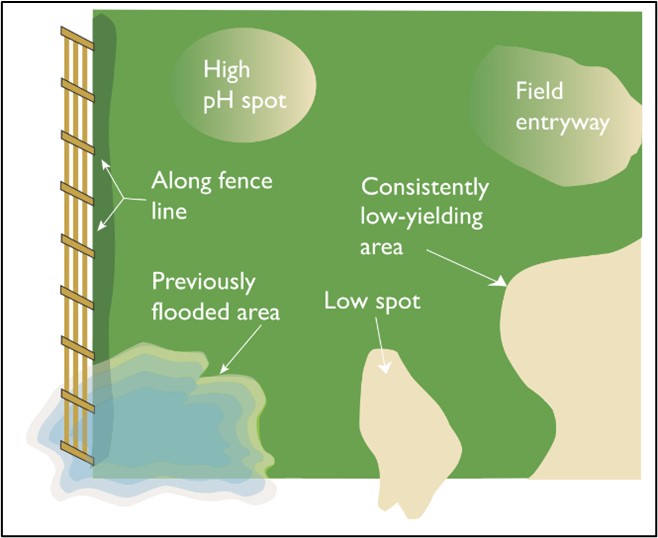
Figure 22. Areas of a field where SCN might first be found and where soybean roots could be checked and soil samples could be collected.
|
 Figure 23. Collecting soil sample in a field of harvested corn in advance of a soybean crop the following growing season. Figure 23. Collecting soil sample in a field of harvested corn in advance of a soybean crop the following growing season.
| 
Figure 24. Collecting soil core for SCN from a soybean field during the growing season.
| 
Figure 25. Collecting soil sample in a field after harvest of soybeans.
|
There are two ways to check fields for SCN. During the growing season, soybean roots can be dug (figure 21), not pulled, from the soil and carefully observed for the presence of tell-tale white SCN females (figure 4 and 5). Roots should be dug no sooner that five to six weeks after planting and no later than about a month before the soybean crop will be harvested. Checking roots of soybeans growing in “high risk" areas where SCN can first appear in a field (figure 22) can make initial checking of fields for the nematode more targeted.
The other way to check for SCN is to collect soil samples before (figure 23), during (figure 24), or after soybeans (figure 25) are grown in a field. These soil samples can be sent to a diagnostic laboratory to determine if SCN is present and, if it is, at what population density. Almost all land-grant universities in soybean-producing states in the United States have laboratories or plant disease clinics that offer this service. There also are numerous private soil testing laboratories that process soil samples for SCN as do a few state departments of agriculture.
Soil samples should consist of multiple soil cores, collected with a soil probe usually 2.5 cm (1 inch) in diameter and 15 to 20 cm (6 to 8 inches) deep, and combined into one composite sample. The soil cores comprising one composite sample should represent an area no larger than 8 hectares (20 acres) and be collected from areas or zones in a field that have common agronomic characteristics (Figure 26). Alternatively, if the field has never been checked for SCN before, the composite soil samples can be collected from “high risk" areas that likely may have had SCN introduced and established (Figure 22). The ideal time to collect composite soil samples for SCN is in the fall, after harvest of crops, unless sampling areas of fields showing obvious symptoms in which samples would be collected during the growing season.
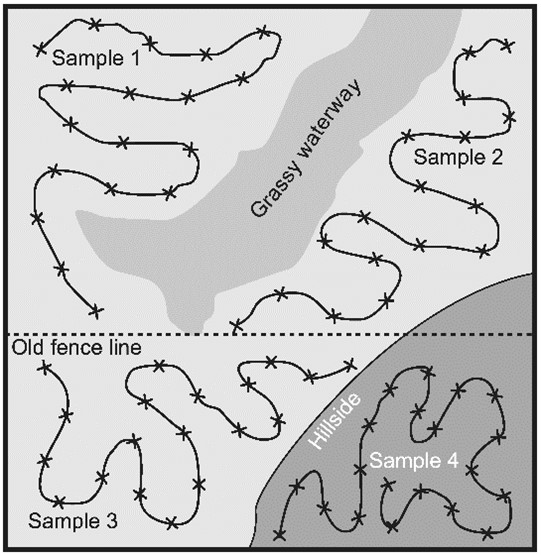
Figure 26. Pattern of soil core collection from four different management zones in a field. Each “x" represents where a single soil core would be collected.
| 
Figure 27. Susceptible (left) and resistant (right) soybean cultivars growing in an SCN-infested field (courtesy S. Chen, Univ. Minnesota)
|
Resistant soybean cultivars
Growing resistant soybean cultivars (varieties) is a primary tool for managing SCN. When effective, resistant cultivars are taller and more robust (figure 27) and produce greater yields than non-resistant (susceptible) cultivars in SCN-infested fields. Currently available resistant cultivars limit the development of SCN adults from second-stage juveniles. Resistance to SCN typically is not 100 percent effective (in other words, the plants are not immune). Plants allowing less than 10 percent SCN reproduction compared to what would occur on a susceptible cultivar typically are considered resistant.
Resistant soybean cultivars traditionally have been developed by taking soybean breeding lines with SCN resistance and crossing them with susceptible soybean cultivars possessing good agronomic traits, including high yield potential. There are seven main breeding lines with SCN-resistance, but almost all SCN-resistant soybean cultivars since 1990 have been developed with the breeding line PI 88788. As a consequence, some SCN populations have developed increased reproduction on these cultivars. In other words, the SCN populations have become “resistant" to PI 88788 resistance.
More soybean cultivars are needed with SCN resistance from breeding lines other than PI 88788 to prolong the effectiveness of resistance by slowing the development of SCN populations that are “resistant to the resistance". This should be achievable as there are already some SCN resistant cultivars available with parentage from breeding lines Peking, PI 437654, and most recently, PI 89772, and BASF Company received approval from the United States Environmental Protection Agency to proceed with development of soybeans with transgenic SCN resistance in 2020.
Nonhost crops
Another primary tool for managing SCN is growing nonhost crops. SCN is an obligate parasite; the nematode is unable to complete its life cycle in the absence of living host roots. Juveniles of SCN that hatch from eggs during years that nonhost crops are grown will perish from starvation or predation or parasitism by other soil-inhabiting organisms. Consequently, SCN population densities decline during any year that nonhost crops are grown.
Soybean is the primary and almost only crop plant grown that is host to SCN. But several types of edible beans in the genus
Phaseolus have been found also to be good hosts for SCN, suffering yield losses and allowing SCN population densities to increase.
The magnitude of decline in SCN numbers during a year that a nonhost crop is grown can vary from year to year and field to field. The decreases in SCN numbers measured when nonhost crops are grown are such that it would take 10 or more years of growing nonhost crops to reduce SCN to undetectable levels or to truly eliminate the pest from the soil. This slow decline is because some SCN eggs are capable of lying dormant (in diapause) in the soil for many years.
SCN-resistant soybean cultivars often are incorporated into multi-year crop rotation sequences with nonhost crops. The combination of these two primary management tools forms an excellent, basic integrated management strategy.

Figure 28. Spring-tooth harrow with soil on the tines.
|
Cultural practices
Maintain a healthy crop. Plants that have adequate moisture and nutrients are better able to withstand infection by SCN. Maintaining proper soil fertility and pH levels and minimizing other plant diseases, insect, and weed pests that weaken the plants are more critical to maximizing soybean yield in fields infested with SCN than those that are not infested.
Manage the movement of soil. If only certain fields on a farm are infested, planting and cultivating of SCN-infested land should be done only after noninfested fields have been worked. Soil on equipment (Figure 28) should be thoroughly removed by power washing, if possible, after working in infested fields.
Soil-applied nematicides
Depending on geographical location, there may be a few soil-applied nematicides labeled for use against SCN. Different products may be labeled for use in different states, and registrations may change from year to year. Users must confirm that nematicides are approved in their state on soybeans in the current growing season before applying any of the products to the soil.
When applied at planting, the effect of the soil-applied nematicides may last long enough to provide an economic yield benefit. However, soil-applied nematicides will not kill all SCN in the soil, and by the end of the growing season SCN numbers may be as high or higher than they were at planting. Also, the efficacy of the nematicide can be affected by soil conditions, temperatures, and rainfall. Yield and economic benefits are not guaranteed, and nematicides are expensive. All nematicides are extremely toxic; only licensed applicators may use these pesticides.
Nematode-protectant seed treatments
Seed-applied products that offer direct or indirect protection of soybean roots from infection by SCN have been available since the mid 2000s. There are numerous nematode-protectant seed treatments available currently (in 2021) and they offer a wide range of chemical and biological active ingredients and modes of action (Table 1). As a group, these products are not all nematicides; some offer protection of soybean roots without directly killing SCN. Some of the seed treatments offer protection against many or all plant-parasitic nematodes and some specifically target SCN. As with soil-applied nematicides, seed treatments likely will not offer season-long protection of roots from SCN but can be effective for a long enough period of time in the beginning of the growing season to result in yield increases.
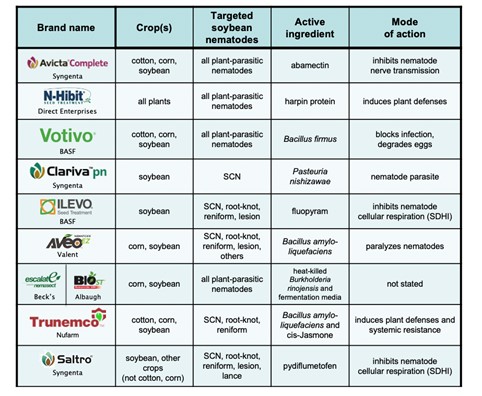
Table 1. Nematode-protectant seed treatments for SCN (current 2021).
|
Cover crops
Cover crops are plants that are seeded into a standing cash crop late in the growing season or after the cash crop is harvested and are grown primarily to reduce or prevent soil erosion and nutrient leaching from bare soil and to increase soil organic matter content. Cover crops have been shown to reduce population densities of several different plant-parasitic nematodes, including some cyst nematode species. Consequently, there is great interest in the possibility that cover crops could reduce population densities of SCN.
Decreases in SCN population densities in soil have been observed in greenhouse and field experiments when some cover crops are grown, but results of other experiments have not shown decreases in SCN population densities. Results from comparable experiments conducted in different fields or in different years by the same investigators have been variable. Presently, it appears that some cover crops can reduce population densities of SCN, but not consistently, and not all plants grown as cover crops have the potential to do so.
Significance
The first documented report of damage by the soybean cyst nematode (Heterodera glycines Ichinohe) was by S. Hori in Japan in 1915. Ancient Chinese literature and personal communications, however, suggest that SCN may have been a pathogen of soybean in China as early as 235 B.C. The term "soybean yellow dwarf" was adopted by Japanese researchers in the 1920s to describe the pale-yellow areas of poor soybean growth observed in fields.
SCN was first discovered in the United States in 1954 in Hanover County, North Carolina – an area known to import flower bulbs from Japan. The rapid spread of SCN to other soybean-growing states (Figure 29) has prompted some to hypothesize that the nematode is indigenous and parasitizing some leguminous weeds in the United States. However, there are indications that soybean seed had been imported to the United States as early as 1765. More significant may be the importation of soil from Asia in the late 1800s to obtain inoculum of the bacterium
Bradyrhizobium japonicum for nitrogen fixation in soybean. Some imported soil was distributed among researchers who were working on the improvement of soybean cultivation in the United States. Soil also was shared among growers to obtain nitrogen-fixing bacteria once its utility had been demonstrated.
The rapid expansion in production acreage of soybean in the United States that started in the mid-1900s served to establish a huge reservoir of host crop for the spread and increase of SCN. A federal quarantine for SCN was established in 1957, but the quarantine was lifted in 1972 because it was ineffective. It has been suggested that the movement of SCN-infested soil and plant material into new soybean production areas already had occurred before the quarantine was established. Currently (2021), SCN has been found in every soybean-producing state in the United States except West Virginia and in many counties and rural municipalities in the provinces of Manitoba, Ontario, and Quebec in Canada (Figure 20), with total soybean yield loss estimates exceeding $1 billion per year. Though SCN has been a recognized pathogen of soybean in the southeastern United States since the 1950s, the detection and effects of SCN in the midwestern United States "soybean belt" was more recently realized.
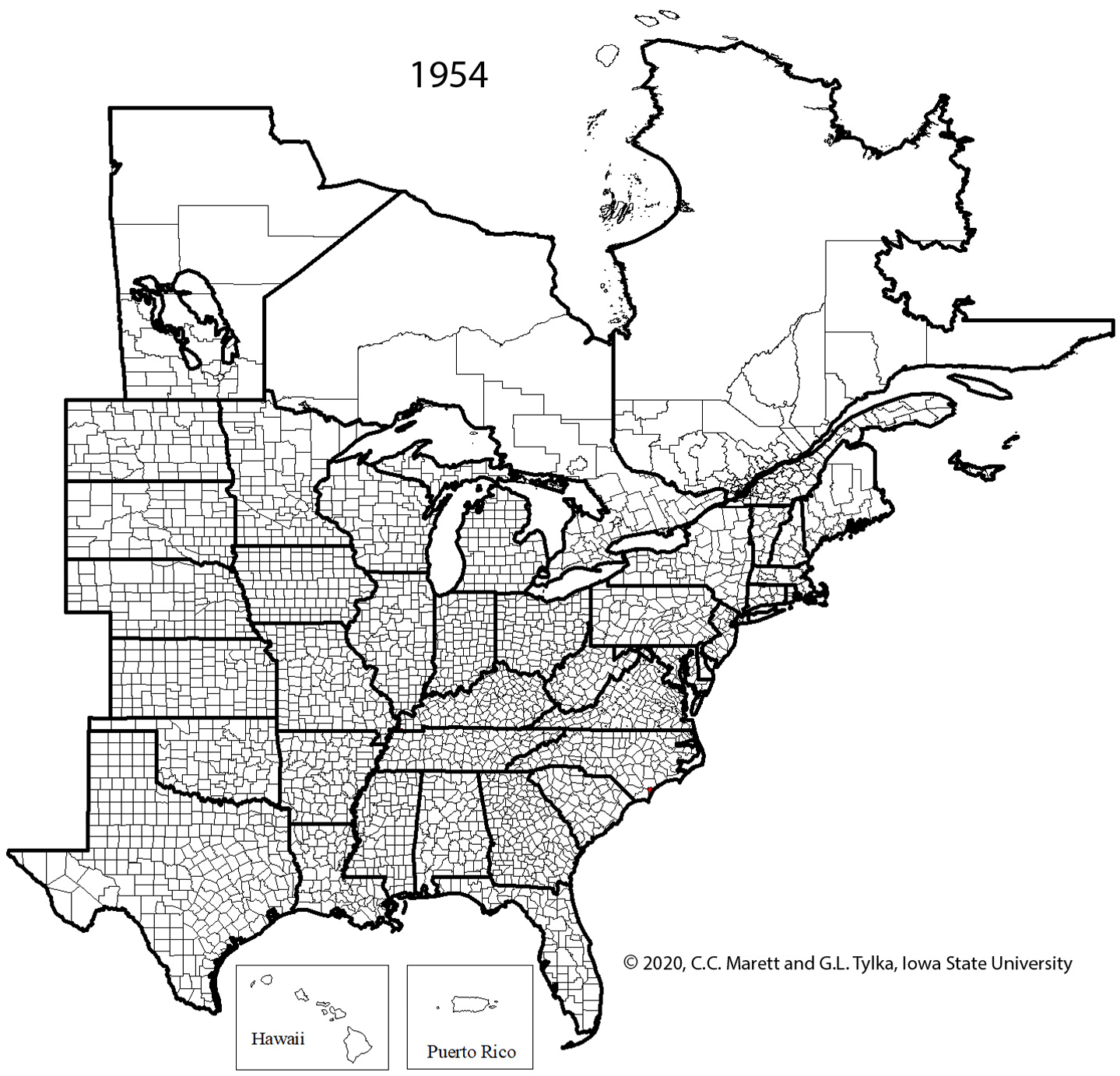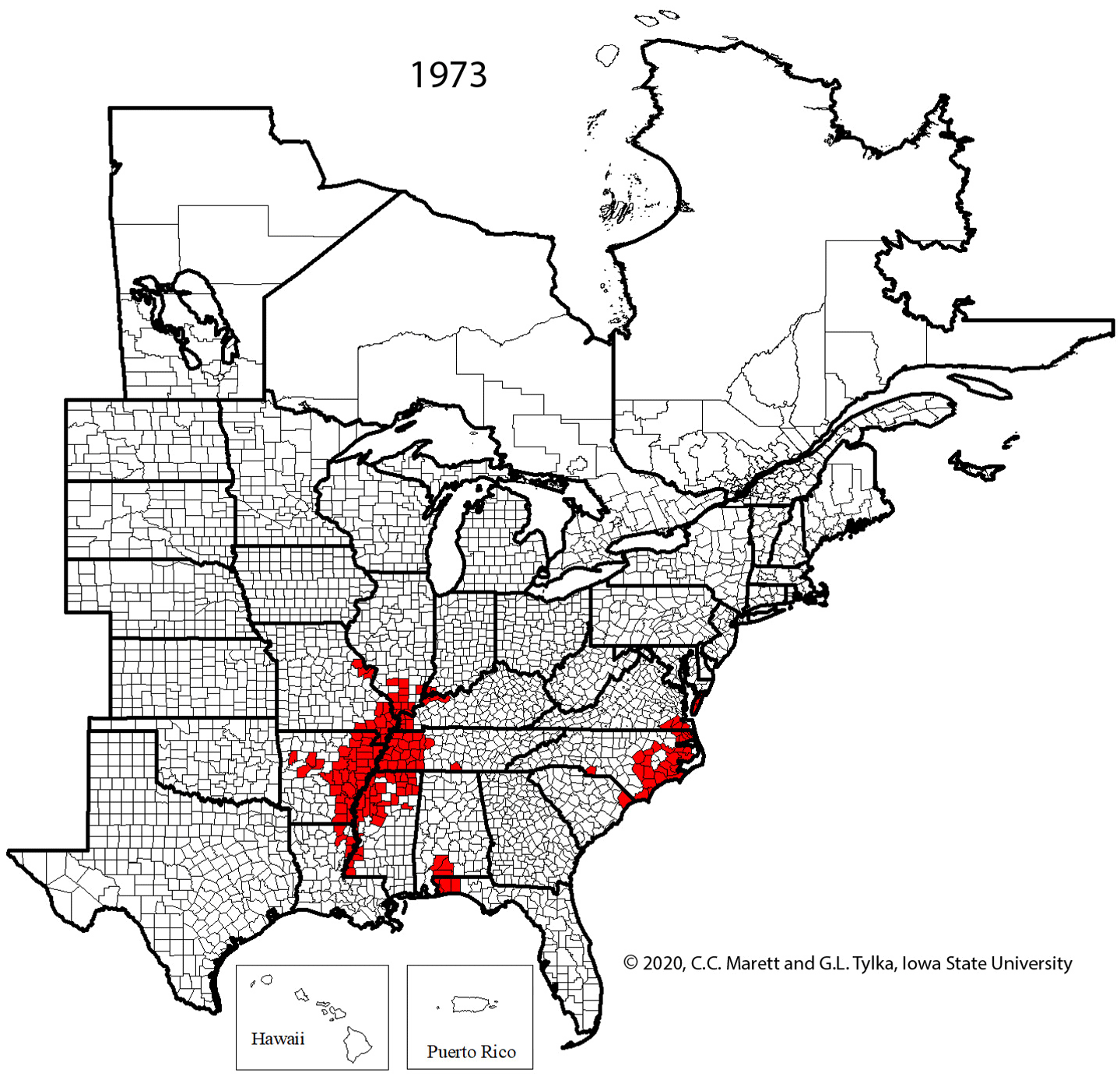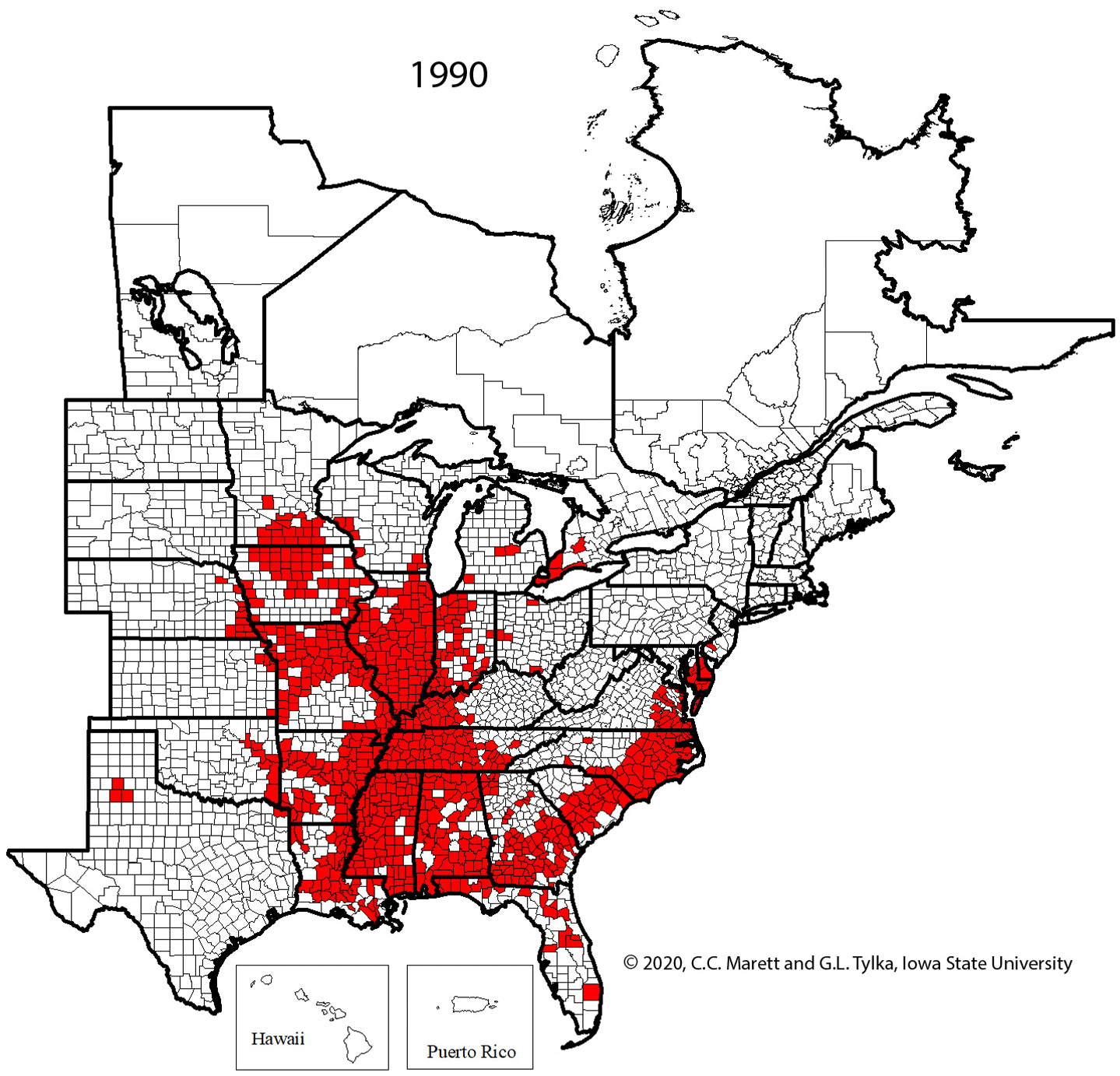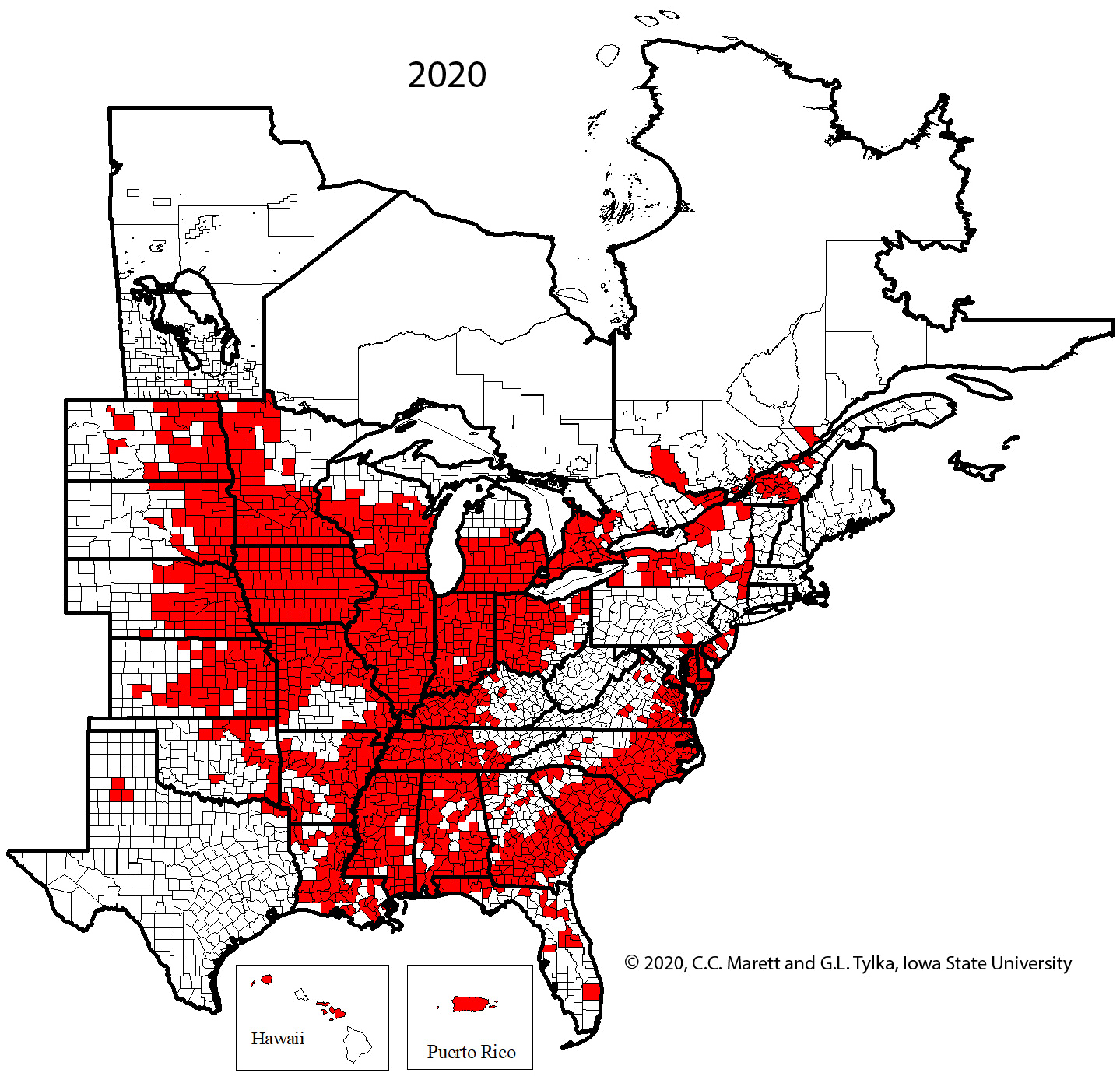
Figure 29. Animated series of maps showing spread of SCN from initial discovery in North Carolina in 1954 to 2020.
SCN was detected in Colombia, South America, in the early 1980s, and was soon thereafter found in Argentina and Brazil - two of the world's important soybean production areas. More recently, SCN was reported from Italy, and if soybean production is expanded in Europe, more SCN infestations likely will be found. In a recent survey of the top ten soybean-producing countries in the world, SCN was found to be the most damaging pathogen of soybean.
Online Resources
There are numerous sources online with information about the biology and management of SCN. Most are targeted towards farmers and those who advise them. Such sites include
www.soybeancyst.info and the SCN section of the Soybean Research and Information Network, see
www.soybeanresearchinfo.com/soybean-disease/soybean-cyst-nematode-scn.
A fairly comprehensive multi-media online resource for high school biology and agriculture teachers and their students that is presented as a realistic, guided case study is “Trouble at Grainly Farms." It can be found at
www.iowapbs.org/static/grainly-farms/home.html
Selected References
Barker, K. R., G. A. Pederson, and G. L. Windham. 1998. Plant and Nematode Interactions. ASA, CSSA, SSA Publishers, Madison, WI.
Davis, E. L. and M. G. Mitchum. 2005. Nematodes: sophisticated parasites of legumes. Plant Physiology 137:1182-1188.
Hartman, G. L., J. C. Rupe, E. J. Sikora, L. L. Domier, J. A. Davis, and K. L. Steffey. 2016. Compendium of Soybean Diseases and Pests, fifth edition. American Phytopathological Society, St. Paul, MN. http://doi.org/10.1094/9780890544754
McCarville, M. C., C. C. Marett, M. P. Mullaney, G. D. Gebhart, and G. L. Tylka. 2017. Increase in soybean cyst nematode virulence and reproduction on resistant soybean varieties in Iowa from 2001 to 2015 and its effects on soybean yields. Plant Health Progress 146-155. http://dx.doi.org/10.1094/PHP-RS-16-0062
Mitchum, M. G. 2016. Soybean resistance to the soybean cyst nematodeHeterodera glycines: an update. Phytopathology 106:1444-1450. http://doi.org/10.1094/PHYTO-06-16-0227-RVWNiblack, T. L., K. N. Lambert, and G. L. Tylka. 2006. A model plant pathogen from the kingdom Animalia: Heterodera glycines, the soybean cyst nematode. Annual Review of Phytopathology 44:283-303. https://doi.org/10.1146/annurev.phyto.43.040204.140218
Schmitt, D. P., J. A. Wrather, and R. D. Riggs (eds.) 2004. Biology and Management of the Soybean Cyst Nematode (Second Edition). Schmitt & Associates of Marceline, Marceline, MO.
Tylka, G.L. and C. C. Marett. 2021. Known distribution of the soybean cyst nematode,Heterodera glycines, in the United States and Canada in 2020. Plant Health Progress. https://doi.org/10.1094/PHP-10-20-0094-BR.
Tylka, G. L. and M. P. Mullaney. 2020. Soybean cyst nematode-resistant soybeans for Iowa. Iowa State University Extension Publication PM 1649, 26 pp. Extension and Outreach Publications. 100. https://store.extension.iastate.edu/product/5154.pdf
Wrather, J. A., T. R. Anderson, D. M. Arsyad, Y. Tan, Y. J. Gai, L. D. Ploper, A. Porta-Puglia, H. H. Ram, and J. T. Yorinori. 2001. Soybean disease loss estimates for the top 10 soybean-producing countries in 1998. Canadian Journal of Plant Pathology 23:115-121.
