Crow, W.T. and Han, H. 2005. Sting nematode. The Plant Health Instructor. DOI: 10.1094/PHI-I-2005-1208-01
Sting nematode
Belonolaimus longicaudatus, Belonolaimus spp.
There are many hosts including common agronomic crops, vegetables, grasses, grains, and trees.
Authors
William T. Crow, Entomology and Nematology Department, University of Florida, Gainesville, FL
Hyerim Han, Applied Entomology Division, National Institute of Agricultural Science & Technology, Rural Development Administration, Suwon, Korea.

Damage caused by sting nematodes
(Belonolaimus longicaudatus)
on strawberry roots. |
Sting nematodes are important ectoparasites of roots of many plant species. Sting nematodes cause significant yield losses in many crops and can lead to complete crop destruction. This nematode-inflicted disease is particularly problematic on golf courses because sting nematodes are prominent in the sandy soils typically used to construct putting greens.
Symptoms and Signs
Plant roots:
Sting nematodes typically feed on root tips. In response to this feeding, root tips cease to grow, causing an abbreviated or stubby-appearing root system (Figures 1, 2, 3, 4). On turfgrasses, the roots may appear cropped-off just below the soil surface (Figure 5). With high population densities, complete root destruction can occur. Damaged roots have a greatly reduced ability to take up water and nutrients from the soil, and this leads to expression of aboveground symptoms and yield reductions. On tuber or root crops, sting nematodes can cause direct damage by causing stunting and malformation of edible portions (Figure 6).

Figure 1 |

Figure 2 |

Figure 3 |

Figure 4 |

Figure 5 |

Figure 6 |
Aboveground Symptoms:
With moderate initial population densities of sting nematodes, young plants may experience reduced vigor, slow growth, and stunting (Figures 7, 8). With high initial population densities, young plants may stop growing completely following seed germination or transplanting (Figures 9A, 9B, 10) and eventually die. The foliage of affected plants may turn yellow or red (Figures 11, 12) due to nutrient deficiencies in the foliage resulting from an impaired root system or to physiological responses in the plant in response to nematode feeding. On corn, brace roots are not able to develop, and the plant is prone to lodging. Chronic wilting is a common symptom of sting nematode damage (Figure 13).

Figure 7 |

Figure 8 |

Figure 9 |

Figure 10 |

Figure 11 |

Figure 12 |

Figure 13 |
On turfgrass, damage by sting nematode usually results in reduced tolerance to drought, wear, and other stresses. This often leads to irregularly shaped patches of wilting, declining turf (Figure 14) and increased development of weeds (Figure 15). Severe infestations may cause turf to die (Figure 16).

Figure 14 |

Figure 15 |

Figure 16 |
Pathogen Biology
Genus Belonolaimus:
Steiner described the first sting nematode Belonolaimus gracilis, which was collected from the rhizosphere of a pine tree (Pinus sp.) in Marion County, FL. Later this nematode was reported to damage various agronomic and horticultural crops in the Atlantic coast states as far north as Virginia. Rau later described B. longicaudatus and identified it as the more common species of Belonolaimus in agricultural settings. Most of the crop damage that was originally attributed to B. gracilis is now recognized as having been caused by B. longicaudatus. Currently there are nine described species of Belonolaimus. Belonolaimus longicaudatus, B. gracilis, B. euthychilus, B. maritimus, and B. nortoni are found in the United States, whereas B. anama, B. jara, B. lineatus, and B. lolii are found elsewhere. Other populations of Belonolaimus that differ from described species by host-range, morphology, and molecular characterization are reported and may represent new species.
Habitat and Distribution:
Belonolaimus longicaudatus is endemic to the coastal plains of the southeastern United States. It has been found as far north along the Atlantic Coast as New Jersey and as far west along the Gulf Coast as Texas, but is most common in Florida. Soil texture is apparently a major factor influencing the distribution of B. longicaudatus both within the soil horizon, and geographically. Reproduction of B. longicaudatus is minimal in soils with < 80% sand content or > 10% clay content. Golf course putting greens are typically constructed with high sand content, providing an ideal habitat for B. longicaudatus. Therefore, it is not surprising that B. longicaudatus has become established outside of its natural geographic range in golf course putting greens in Australia, the Bahamas, Bermuda, and California through introduction on infested planting material.
Morphology and Identification:
Sting nematodes are among the largest plant-parasitic nematodes, up to 3 mm long. The stylet of B. longicaudatus is long and thin, and the anterior region of the head is offset by a constriction (Figure 17). The median bulb of the esophagus is very pronounced, and the esophageal glands overlap the intestine. Female B. longicaudatus have the vulva located near the middle of the body (Figure 18) and have a rounded tail (Figure 19). Males have a long tail that narrows dorsally to a point, well developed spicules, and a small bursa (Figure 20). Juvenile stages look very similar to adults (Figure 21).
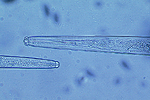
Figure 17 |
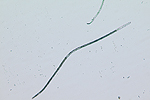
Figure 18 |
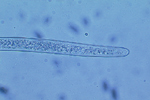
Figure 19 |
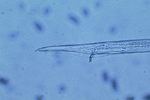
Figure 20 |

Figure 21 |
Feeding and host range:
Sting nematodes are ectoparasites, meaning that they typically feed with their bodies outside of plant tissue. To feed, sting nematodes insert their long stylet into tissue near the root tip and then ingest the contents of the root cells through it. After feeding on a root tip, a sting nematode can withdraw its stylet and move to another feeding site. Feeding by multiple sting nematodes on the same root tip (Figure 22) can cause root growth to cease and lead to a proliferation of lateral roots. The nematodes then feed on and damage these developing lateral roots, giving rise to a bunchy or stubby appearance.

Figure 22 |
The host range of B. longicaudatus is extensive and includes vegetables (e.g., beans, carrot, corn, crucifers, potato), fruits (e.g., citrus, strawberry), agronomic crops (e.g., cotton, peanut, sorghum, soybean), turfgrasses (e.g., bermudagrass, St. Augustinegrass, zoysiagrass) and forest crops (pine trees). Several studies suggest the existence of physiological races of B. longicaudatus. Physiological races are populations of a given species that show different host preferences than other populations of the same species.
Disease Cycle and Epidemiology
Epidemiology
The life cycle of B. longicaudatus has been studied on roots of corn (Zea mays) in axenic root culture. Like other plant-parasitic nematodes, the sting nematode begins life as an egg. Figure 24 shows the process of embryogenesis of B. longicaudatus from the one-celled stage to a first-stage juvenile (J1) that molts inside the egg into a second-stage juvenile (J2) ready to hatch. After hatching, the J2 move through soil to the root system of a host plant where they congregate around root hairs. The J2 feed on the root hairs until they molt into third-stage juveniles (J3). The J3 move immediately to the meristems of either major or lateral roots to feed, as do all subsequent life-stages. The J3 molt to become fourth-stage juveniles (J4), which molt to become adults.
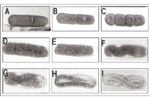
Figure 24 |
Sting nematodes are amphimictic, meaning that mating is required to produce viable offspring. Therefore, both male and female nematodes are abundant in most populations. Males and females are attracted to each other for reproduction (Figure 25 A, B). Female sting nematodes have a spermatheca, a sperm-storage organ, so only a single mating is required for fertilization of many eggs over time. Eggs are produced separately from each of two paired ovaries, and a single female was observed to lay nine to ten eggs over a 10 to 15 hour period (Figure 26).

Figure 25 |

Figure 26 |
Sting nematodes have no long-term survival stage, so populations decline rapidly in the absence of a host. A population decline model for a population of sting nematodes in northern Florida showed a negative exponential decline in numbers during clean fallow, resulting in a 95% decrease after 200 days. Because females can begin laying eggs immediately after feeding, populations can increase rapidly when hosts are planted. However, the virulence of B. longicaudatus may limit population increases. High numbers of sting nematodes at planting can inhibit root development to the extent that food is limited and nematode populations cannot increase beyond a certain level. This is a phenomenon known as a carrying capacity, the maximum number of nematodes that can be sustained on a given host under a given set of conditions. The carrying capacity for a population of B. longicaudatus on cotton in northern Florida was approximately 140 nematodes per 130 cm3 of soil.
Disease Management
Chemical management
Sting nematodes can be effectively managed with nematicides. Unlike many of the endoparasitic nematodes that spend a majority of their life within roots, contact nematicides often work well on sting nematode. Both carbamate (aldicarb, carbofuran) and organophosphate (fenamiphos, ethoprop, turbufos) nematicides and fumigants (methyl bromide, 1,3-dichloropropene, metam sodium) are currently registered and can be effectively used to reduce sting nematode populations. On annual crops, nematicides applied either before or at planting usually provide acceptable levels of control by protecting newly developing root systems. On perennial crops such as turfgrasses, seasonal application of post-plant nematicides during times of root growth may be required.
Cultural practices
When possible, avoid use of infested planting material. Most warm-season turfgrasses are planted as sod or sprigs, so sting nematodes and other pathogens can be moved in the soil adhering to the sod (Figure 27). It is believed that this is the primary way that the sting nematode has become established in new areas, especially outside of its native geographical range.

Figure 27 |
On turfgrasses, relieving additional stresses by raising mowing height, increasing irrigation frequency, improving aeration to roots, and reducing traffic can improve tolerance to sting nematodes. The addition of organic, and some inorganic, amendments to soil also can improve tolerance to sting nematodes by improving the water and nutrient-holding capacity of the soil. Organic amendments have also been shown to reduce population densities of sting nematodes in some studies. This may be due to direct effects of these additives on the nematodes or due to stimulation of antagonistic microorganisms in the soil.
Biological control
Pasteuria usgae, an endospore-forming bacterium, is an obligate parasite of B. longicaudatus (Figures 28, 29). This bacterium is found in soils throughout Florida, and presumably other areas where sting nematodes occur. Pasteuria usgae was successfully introduced into a previously non-infested putting green resulting in the suppression of sting nematodes. Presently, the only method for infesting a field site with P. usgae is by adding soil from a site that already has sting nematodes infected with the bacterium. Unfortunately, this method is not economically feasible for commercial use. However, in vitro production of P. usgae is being attempted at this time. If these efforts are successful, P. usgae may become a viable inoculative biological control agent for sting nematodes in the future.
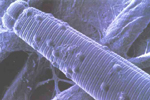
Figure 28 |
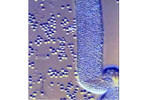
Figure 29 |
Some researchers have found that application of various entomopathogenic nematodes (nematodes used for biological control of insect pests) on turf can reduce sting nematode populations. However, research trials conducted in Florida have revealed that the results are too inconsistent to be relied on as a management tool in that state.
Significance
Sting nematodes cause yield losses in many crops and can lead to complete crop destruction. On many crops, the economic threshold for use of control measures is at, or near, the detection level. Fortunately, B. longicaudatus, the most damaging species of sting nematode, is restricted to sandy areas and is primarily a problem in Florida and coastal plain areas of other southeastern states. However, due to movement on infested planting material and the high sand content in most putting greens, B. longicaudatus is becoming more common on golf courses outside of its native geographic range. Beyond the damage they cause to turfgrass, sting nematodes also can have a negative impact on water quality and quantity by requiring increased irrigation frequency and increasing the likelihood of nitrates leaching into ground water.
Selected References
Abu-Gharbieh, W.I., and V.G. Perry. 1970. Host differences among populations of Belonolaimus longicaudatus Rau. J. Nematology 2:209-216.
Crow, W. T., D. P. Weingartner, R. McSorley, and D. W. Dickson. Population dynamics of Belonolaimus longicaudatus in a cotton production system. J. Nematology 32:210-214.
Han, H. 2001. Characterization of intraspecific variations of Belonolaimus longicaudatus by morphology, developmental biology, host specificity, and sequence analysis of its ITS-1 rDNA. Ph.D. Dissertation, University of Florida, Gainesville, FL.
Huang, X., and J. O. Becker. 1999. Lifecycle and mating behavior of Belonolaimus longicaudatus in gnotobiotic culture. J.Nematology 31:70-74.
Huang, X., and J. O. Becker. 1997. In vitro culture and feeding behavior of Belonolaimus longicaudatus on excised Zea mays roots. J. Nematology 29:411-415.
Smart, G.C. and K.B. Nguyen. Sting and awl nematodes. Pp. 627-668 in W.R. Nickle ed., Manual of Agricultural Nematology. Marcel Dekker Inc., NY.
Perry, V.G., and H. Rhoades. 1982. The genus Belonolaimus. Pp. 144-149 in R.D. Riggs ed., Nematology in the southern region of the United States. Southern Cooperative Series Bulletin 276. Fayetteville, AR: University of Arkansas Agricultural Publications.
Robbins, R.T., and K.R. Barker. 1973. Comparisons of host range and reproduction among populations of Belonolaimus longicaudatus from North Carolina and Georgia. Plant Dis. Reporter 57:750-754.
