Tan spot of cereals
Pyrenophora tritici-repentis
small grain cereals, grasses

Tan spot lesions on wheat.
Refer to the Tan Spot Glossary for definitions of terms.
Tan spot of wheat (Triticum aestivum), also known as yellow leaf spot, is caused by the fungus Pyrenophora tritici-repentis (asexual stage: Drechslera tritici-repentis) and occurs worldwide wherever wheat and other susceptible host crops are grown. The disease develops on wheat in the spring and summer on both the upper and lower surfaces of leaves. The fungus also can infect wheat spikes and eventually the kernels, causing a disease of the seeds known as red smudge. In North America, the complex of foliar diseases that includes tan spot has increased over the last several decades due to wheat culture in the same field year after year and a shift toward conservation tillage practices that leave crop residue on the soil surface. In addition to tan spot, this complex includes diseases such as Septoria tritici blotch (Mycosphaerella graminicola) (asexual stage: Septoria tritici), spot blotch (Cochliobolus sativus) (asexual stage: Bipolaris sorokiniana), and Stagonospora nodorum blotch (Phaeosphaeria nodorum) (asexual stage: Stagonospora nodorum). When wheat residue is left on the soil surface, as in minimum- or no-tillage, tan spot often becomes the predominant disease.
Symptoms and Signs
Symptoms can be manifested as necrosis or chlorosis or both. The necrosis symptom comprises spots that appear initially as tan-brown flecks and expand into lens-shaped, tan lesions with yellow borders (Figure 1). The chlorosis symptom consists of rapidly expanding yellow areas surrounding lesions on the leaf blades. Lesions may coalesce into large blotches as they age, predisposing leaves to premature senescence (Figure 2). If infected leaves are moistened, the lesions darken at the center (Figure 3) due to formation of conidiophores and conidia of D. tritici-repentis. Other pathogens such as M. graminicola, P. nodorum, and C. sativus can cause similar lesions on leaves. Positive identification of tan spot is achieved by examining the morphology of the conidia formed in lesions. Dark, erumpent sexual fruiting structures of P. tritici-repentis known as pseudothecia (Figure 4) develop and mature on wheat straw in the fall and winter. Symptoms on the wheat spike are not easily distinguished and may include bleached or brownish glumes. Infected kernels are characterized by a reddish color on the seed coat (red smudge, Figure 5).

Figure 1 |

Figure 2 |

Figure 3 |

Figure 4 |

Figure 5 |
Pathogen Biology
Classification and host range
P. tritici-repentis is classified as follows: Kingdom: Fungi; Phylum: Ascomycota; Class: Dothidiomycetes; Subclass: Pleosporomycetidae; Order: Pleosporales; Family: Pleosporaceae; Genus: Pyrenophora; Species: tritici-repentis. P. tritici-repentis has a wide host range that includes several cultivated grass hosts, such as T. aestivum (bread wheat), T. turgidum (durum wheat), Hordeum vulgare (barley), and Secale cereale (rye), but also many other grass species.
Spores
P. tritici-repentis produces two types of spores. Asexual spores known as conidia are produced mainly in maturing lesions on leaves but may also be produced on other plant parts. Conidiophores (Figure 6, black arrow) are erect, simple (not branched), have a swollen base, and are olive-black in color. Conidia (Figure 6, 7) are hyaline (non-pigmented) to light brown, cylindrical, and have one to nine transverse septa (Figure 7). The conical tapering of the basal cell of the conidium (Fig.7, arrow), which gives the appearance of a snake head, is diagnostic of D. tritici-repentis. Sexual spores known as ascospores are produced in pseudothecia. Each pseudothecium contains saclike cells called asci (Figure 8) in which meiosis followed by mitosis occur leading to formation of eight ascospores per ascus. Mature ascospores are yellow-brown and oval to globose. Each mature ascospore has three transverse septa, and the median cells may have a longitudinal septum (Figure 9, arrow).
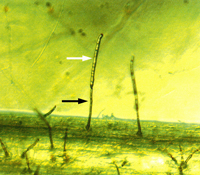
Figure 6 |
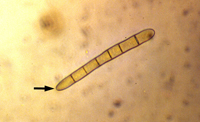
Figure 7 |
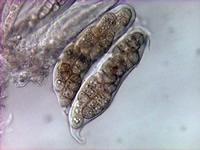
Figure 8 |
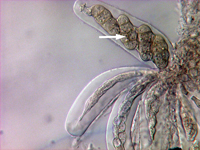
Figure 9 |
Growth on agar media
P. tritici-repentis grows on potato dextrose agar, forming a greenish-gray, dense, fluffy mycelium which does not produce conidia. On V8 agar, it forms a white to light gray mycelium, which produces conidiophores and conidia after exposure to near-ultraviolet light for 12 to 24 hours followed by 12 to 24 hours of darkness.
Physiologic races
The observation that individual isolates of P. tritici-repentis induced either necrosis or chlorosis independently in specific cultivars or wheat lines initially prompted researchers to classify isolates of the pathogen into four pathotypes. The pathotypes were based on the symptoms that a particular isolate would produce in selected differential cultivars or lines of wheat. Pathotype 1 produced necrosis and chlorosis, pathotype 2 necrosis only, pathotype 3 chlorosis only, and pathotype 4 produced neither necrosis nor chlorosis.
Later, isolates were identified that caused chlorosis on cultivars or lines that were previously known to be resistant to all isolates, which led to the introduction of a race identification scheme. At least eight races of P. tritici-repentis have been characterized according to their virulence patterns on a set of three differential wheat genotypes: the cultivar ‘Glenlea,’ and lines 6B365 and 6B662. Race 4 does not attack any of the differentials. Races 2, 3, and 5 are considered the “basic” races because they can attack only one of the three differentials. Races 1, 6, and 7 can attack two differentials, that is, they combine the virulence of two basic races. Race 8 can attack all three differentials; therefore it combines the virulence of all three basic races. The number of races is expected to increase as the differential set is expanded and new isolates are tested. In North America, races 1 and 2 are the most commonly detected. Race 1 is the most prevalent in the Great Plains of the United States.
Toxins
The interaction of virulent races of P. tritici-repentis with host lines is highly specific. A host-specific toxin mediates the interaction between a given race and its susceptible differential host line. Three toxins have been identified and demonstrated to be pathogenicity factors. Ptr ToxA induces the necrosis symptom. The other two toxins, Ptr ToxB and Ptr ToxC, both induce chlorosis, but on different host lines and cultivars
Recently, production of the anthraquinones catenarin and emodin was demonstrated in wheat tissues inoculated with P. tritici-repentis. Catenarin is a red pigment hypothesized to cause the red smudge symptom in infected kernels. It also induces non-specific necrosis on wheat leaves, implying that it plays a role in non-specific symptom development by P. tritici-repentis. It also has been shown to be toxic to some of the fungi associated with P. tritici-repentis during its saprophytic and parasitic stages of growth, and may therefore play a role in the life strategy of the pathogen. Emodin is a direct precursor of catenarin and has been classified as a diarrhea-inducing, genotoxic, mutagenic, and cytotoxic mycotoxin in mammals.
Disease Cycle/Epidemiology
Survival
P. tritici-repentis survives intercrop periods as pseudothecia on host residue, which is considered the main source of primary inoculum. Other sources of inoculum mainly in the form of conidia include infected seed, volunteer wheat, and other grass species.
Disease cycle
The disease cycle is illustrated in Figure 10. Pseudothecia develop and mature on wheat straw in the fall and winter. In the spring, ascospores are discharged from pseudothecia and cause primary infections on leaves. They are dispersed by wind. Due to their large size, their dispersal is only over short distances (several centimeters). Ascospore discharge is favored by rainfall, high relative humidity, and temperatures above 10°C (50°F). Conidia produced in maturing lesions on leaves serve as secondary inoculum. However, conidia can also be produced on straw and serve as primary inoculum. Conidia are produced in larger numbers and, due to their light weight, are dispersed by wind over longer distances (kilometers to tens of kilometers) than ascospores. Hence, they are epidemiologically more important than ascospores. During and after maturation of the wheat crop, the fungus grows saprophytically as mycelium from the infected leaf blade down the leaf sheath and onto the stem where it will later form pseudothecia.
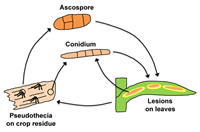
Figure 10 |
Infection process
When an ascospore or a conidium of P. tritici-repentis lands on a leaf of a susceptible host, it germinates in response to free moisture by forming a germ tube (Figure 11). The germ tube produces an appressorium and a penetration peg (Figure 11).&) that enters epidermal cells directly or through stomates and forms a vesicle. Fungal growth develops from the vesicle and proceeds intercellularly in the mesophyll layer (Figure 12). Damage of cellular organelles beyond the advancing hyphae implies the involvement of a toxin in the infection process. At 20°C (68°F), most infections occur between 6 and 24 hours in the presence of free moisture.
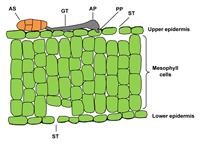
Figure 11 |
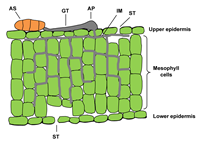
Figure 12 |
Infections of a wheat crop are most numerous when pathogen-infested straw is present on the soil surface within or adjacent to the crop (Figure 13). Rainfall favors conidial production and release as well as lesion expansion. Severe epidemics and yield loss can result when wet weather prevails during the growing season.

Figure 13 |
Disease Management
Effective management of tan spot is achieved by integrating available strategies.
Cultural management
Tan spot can be managed by cultural practices such as crop rotation with non-hosts, removal or destruction of infested residue, or tillage, which buries infested residue. Examples of non-host crops include mustard, flax, crambe, and soybean. Although corn is a non-host for P. tritici-repentis, planting wheat into corn residue may increase the risk of another disease known as Fusarium head blight or scab.
Resistant cultivars
Planting resistant cultivars is one of the least expensive and most effective management strategies for tan spot. Durum and bread wheat cultivars with resistance to tan spot races prevalent in the U.S. are available. However, resistant cultivars are not currently available in other wheat types.
Foliar fungicides
Fungicides in the strobilurin and triazole classes provide very good to excellent control of tan spot. They include azoxystrobin, pyraclostrobin, metconazole, propiconazole, prothioconazole, tebuconazole, prothioconazole + tebuconazole, metconazole + pyraclostrobin, propiconazole + azoxystrobin, and propiconazole + trifloxystrobin. Benefit (yield loss reduction) from fungicide application is greatest when disease pressure is severe, e.g., when wheat is planted into wheat stubble, a susceptible cultivar is grown, or an environment favorable to disease development, such as prolonged rainfall, prevails during the growing season. The optimum fungicide application timing to protect the flag leaf is between Feekes growth stage 10 (boot) and Feekes 10.5 (fully headed). However, an earlier application may be necessary if the onset of tan spot is early in the growing season and environmental conditions, cultivar susceptibility, and tillage type favor development of damaging levels of disease. In addition to tan spot, other diseases may need to be controlled. Therefore, fungicide applications at a growth stage later than Feekes 10 to Feekes 10.5 may be needed to achieve acceptable disease control. Because two applications in a single growing season may not be economical, often the producer is faced with the task of deciding which disease is most important to control at what growth stage.
Seed treatment fungicides
Because P. tritici-repentis can be seed-transmitted, treating seed with fungicide before planting can reduce seed-borne inoculum. Several systemic fungicides are available and can be used individually or in combination to treat seed. They include difenoconazole, thiram, triticonazole, and carboxin. However, the seedborne phase is usually not important epidemiologically, and seed treatments for the management of tan spot by itself would not be recommended. Seed is often treated to control diseases such as loose smut, common bunt, covered smut, damping off, and early season powdery mildew and leaf rust.
Significance
Tan spot has the potential to significantly reduce yield and hence profitability for wheat and other small grain growers. Yield losses of up to 50% have been documented. In areas or regions where continuous wheat culture is practiced, susceptible cultivars are grown, and minimum or no tillage is practiced, tan spot is one of the major constraints in commercial wheat production.
Acknowledgments
Reviewers: W. Bockus and M. McMullen.
Images contributed: M. McMullen, J. Krupinsky, and W. Bockus.
Selected References
Agrios, G.N. 2005. Plant Pathology. Fifth Edition. Elsevier, New York, NY.
Bockus, W.W., and M.M. Claassen. 1992. Effects of crop rotation and residue management practices on severity of tan spot of winter wheat. Plant Disease 76:633-636.
Bouras, N., and S.E. Strelkov. 2008. The anthraquinone catenarin is phytotoxic and produced in leaves and kernels of wheat infected by Pyrenophora tritici-repentis. Physiological and Molecular Plant Pathology 72:87-95.
Campbell, N.A., J.B. Reece, and L.G. Mitchell. 1999. Biology. Fifth Edition. Benjamin/Cummings, Menlo Park, CA.
Carigano, M., S.A. Staggenborg, and J.P. Shroyer. 2008. Management practices to minimize tan spot in a continuous wheat rotation. Agronomy Journal 100:145-153.
De Wolf, E.D., R.J. Effertz, S. Ali, and L.J. Francl. 1998. Vistas of tan spot research. Canadian Journal of Plant Pathology 20:349-370.
Duveiller, E., Y.R. Kandel, R.C. Sharma, and S.M. Shrestha. 2005. Epidemiology of foliar blights (spot blotch and tan spot) of wheat in the plains bordering the Himalayas. Phytopathology 95:248-256.
Editorial Staff. 2001. Random House Webster's Unabridged Dictionary. Second Edition. Random House, New York, NY.
Krupinsky, J. M. 1992. Grass hosts of Pyrenophora tritici-repentis. Plant Disease 76:92-95.
Lamari, L., B.D. McCallum, and R.M. dePauw. 2005. Forensic pathology of Canadian bread wheat: the case of tan spot. Phytopathology 95:144-152.
McMullen, M., and T. Adhikari. 2009. Fungal Leaf Spot Diseases of Wheat: Tan Spot, Stagonospora nodorum blotch and Septoria tritici blotch. PP-1249. North Dakota State University Extension Service.
McMullen, M.P. 2010. Tan spot (yellow leaf spot). Pages 82-84 in: Compendium of Wheat Diseases and Pests. W.W. Bockus, R.L. Bowden, R.M. Hunger, W.L. Morrill, T.D. Murray, and R.W. Smiley, eds. American Phytopathological Society, St. Paul, MN.
Murray, T.D., D.W. Parry, and N.D. Cattlin. 1998. A Color Handbook of Diseases of Small Grain Cereal Crops. Iowa State University Press, Ames, IA.
Rees, R.G., G.J. Platz, and R.J. Mayer. 1982. Yield losses in wheat from yellow spot: comparison of estimates derived from single tillers and plots. Australian Journal of Agricultural Research 33:899-908.
Shurtleff, M.C., and C.W. Averre III. 1997. Glossary of Plant-Pathological Terms. APS Press, St. Paul, MN.
Singh, S., W.W. Bockus, I. Sharma, and R.L. Bowden. 2008. A novel source of resistance in wheat to Pyrenophora tritici-repentis race 1. Plant Disease 92:91-95.
Strelkov, S.E., and L. Lamari. 2003. Host-parasite interactions in tan spot [Pyrenophora tritici-repentis] of wheat. Canadian Journal of Plant Pathology 25:339-349.
Tadesse, W., S.L.K. Hsam, and F.J. Zeller. 2006. Evaluation of common wheat cultivars for tan spot resistance and chromosomal location of a resistance gene in the cultivar ‘Salamouni’. Plant Breeding 125:318-322.
Wegulo, S.N., J.A. Breathnach, and P.S. Baenziger. 2009. Effect of growth stage on the relationship between tan spot and spot blotch severity and yield in winter wheat. Crop Protection 28:696-702.
