White pine blister rust
Cronartium ribicola (Peridermium strobi)
White (five-needle) pines (Pinus spp.) and currants and gooseberries (Ribes spp.).

Aeciospores are released
from a canker on a western
white pine after peridia rupture.
Symptoms and signs
White pine blister rust causes a range of symptoms on the white pine hosts. The earliest symptoms are indistinct chlorotic spots on needles shortly after infection. As the fungus moves into the branches, spindle-shaped swellings form (Figure 1) and eventually develop into cankers. The bark at the margins of these cankers often has an orange color (Figure 2). When the fungus girdles a branch, that branch dies within a few years and the dead needles on the affected branch turn red. This is called a blister rust "flag" (Figure 3) and often is the first indication of disease in the forest or plantation. If the branch is not killed, the fungus may continue to grow into the trunk, or bole, causing a canker that can kill that portion of the tree upward. Cankers often exude large amounts of resin (Figure 4), another conspicuous diagnostic symptom. Sometimes, rodents eat the bark at the canker margin, as seen in Figure 4.

Figure 1 |

Figure 2 |

Figure 3 |

Figure 4 |
Signs of the disease on pine occur as the fungus develops and produces two of the five spore stages characteristic of the rust. Pycnia develop at the margin of a canker a year or two after the canker forms. This stage is apparent for a short time in the spring and appears as small honey-colored droplets on the surface of the bark, consisting of masses of pycniospores (spermatia). Aecia develop in this region of the canker the following year. This sequence of pycnial and aecial development occurs progressively each year as the canker grows and continues as long as the fungus remains active. The aecial pustules erupt through the bark of cankers and are covered at first by a white membrane, the peridium (Figure 5). The peridium soon ruptures to release masses of yellow-orange aeciospores (Figure 6). This stage gives the disease the name "blister rust."

Figure 5 |

Figure 6 |
Symptoms on the alternate hosts, currants and gooseberries (collectively referred to as Ribes spp.), are not as distinct as on the pines and usually consist of chlorotic spots on the upper leaf surface. If leaves are severely infected the leaves may drop prematurely, but usually there is little damage to these host plants. Signs of the disease on leaves of Ribes are orange pustules that develop on the underside of leaves below the chlorotic spots (Figure 7). This is the uredinial stage of the fungus in which masses of orange urediniospores are produced. As the season progresses, brown, hair-like telial columns (Figure 8) develop from the uredinial pustules.
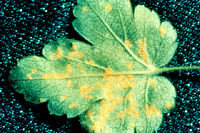
Figure 7 |

Figure 8 |
Pathogen Biology
The white pine blister rust pathogen is a typical heteroecious, macrocyclic rust that produces five distinct spore stages on two different hosts to complete its life cycle. The pycnial stage consists of pycniospores, or spermatia, which are haploid spores that fertilize compatible receptive hyphae. The two sexes are not distinguishable and are simply designated plus and minus. This is the stage where genetic recombination can occur that may lead to development of races of the rust. However, the nuclear cycle (i.e., dikaryogamy, diploidization, meiosis) of the blister rust fungus has not been fully determined, but is assumed to be the same as for other better known rust fungi such as Puccinia graminis. The aecial stage develops in host tissue occupied by pycnia the previous season (Figure 6). The fungus is perennial in the pine host and aeciospores are produced annually as long as the host tissues remain alive. Aeciospores are disseminated by wind over long distances, and Ribes spp. as far as 480 km (300 miles) from the nearest known white pines have been infected.

Figure 6 |
Within a few weeks after infection of Ribes, the uredinial stage (Figure 7) appears and urediniospores continue to be produced until the end of the growing season . Uredinia comprise the repeating stage of the fungus, and urediniospores infect other Ribes in the area. Teliospores, which make up the telial columns (Figure 8), germinate to produce a four-celled promycelium on which four haploid basidiospores (sporidia) develop. The basidiospores are small, thin-walled and relatively short-lived. They are wind-borne to pines to start the disease cycle.

Figure 7 |

Figure 8 |
Pathogenic races are common in rust fungi, and early European pathologists suggested the occurrence of races of the white pine blister rust fungus. The occurrence of pathogenic variation, specifically physiologic races, has been based on several criteria but not yet by the traditional method of inoculating differential hosts and determining if infection occurs.
The blister rust fungus is believed to have originated in Asia on pines native to that region but did not become a serious problem until after the highly susceptible American white pines were introduced into Europe as early as the 16th century. Eastern white pine seed from America was planted at "Longleat," the estate of Lord Weymouth at Wiltshire, England, and soon was planted throughout Europe where this pine is known as Weymouth pine. The disease on white pine was considered to be the same as a stem rust that occurs on European pines such as Scotch pine, and the fungus was called Peridermium pini. In the late 1800s Heinrich Klebahn separated this fungus group into three species and renamed the white pine blister rust fungus Peridermium strobi. The fungus on Ribes spp. was first reported in Europe in the mid 1800s, and named Cronartium ribicola. "Ribicola" means living on Ribes. Klebahn proved the connection of the two fungal stages in 1889 by dusting Ribes leaves with aeciospores from pine and completed the cycle a few years later by placing healthy white pine seedlings in a bell jar with infected Ribes bushes. Peridermium refers only to the stages on pine, and Cronartium ribicola is now the name used for the heteroecious, macrocyclic rust pathogen found on both pines and Ribes.
Disease Cycle and Epidemiology
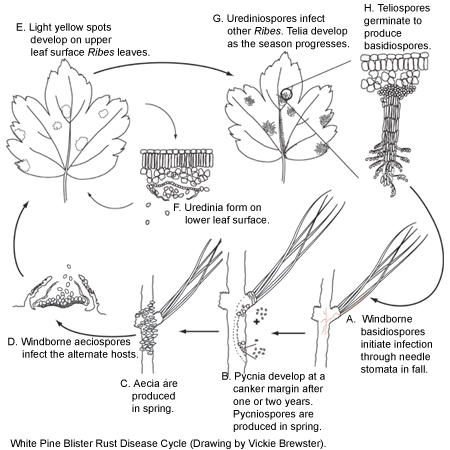
Figure 9 |
The blister rust cycle (Figure 9) starts in the fall when pine needles are infected by basidiospores from the alternate host (Ribes spp.) (Figure 9A). The basidiospores germinate, and the fungus mycelium enters the needles through stomata and grows down the needle into the branch. The fungus continues to develop between the cells of the inner bark, and nutrient absorbing haustoria penetrate into the phloem cells. The mycelial network continues to develop both lengthwise and laterally in a spindle-shaped pattern to produce a swollen canker (Fig. 9B). In the spring of the second year, pycnia are produced in the margins of the infected area. The following year aecia are produced and erupt through the bark where pycnia were produced the previous year (Figure 9C). This disruption of the bark leads to drying and death of the inner bark and this dead area enlarges over several years to become a blister rust canker. The two or three year latent period between initial infection and appearance of symptoms or signs allows the disease to become established in a forest stand before it is detected. Aeciospores (Figure 9D) can survive for several months and are wind-borne for long distances where they infect the alternate hosts (Ribes spp.).
A few weeks after the Ribes are infected, light yellow spots develop on the upper leaf surface (Figure 9E) and uredinia (Figure 9F) develop on the undersides of infected leaves. This is the repeating stage of the rust, and urediniospores infect other Ribes bushes in the general area as far as 1.6 km (one mile) distant, perhaps farther. Telial columns also develop on the underside of leaves (Figure 9G), usually from uredinial pustules, and may start forming soon after the uredinia appear, increasing in number as the season progresses. The individual teliospores that make up the telial column germinate (Figure 9H) to produce basidiospores that infect the pine hosts, completing the disease cycle.
Blister rust infection is favored by cool, moist conditions, and the disease tends to be more prevalent in low-lying areas such as creek bottoms, swampy depressions and openings in timber stands. Besides providing conditions favorable for infection, air currents carrying basidiospores are more likely to flow into and settle in these depressions. Studies in the Great Lakes Region and elsewhere have recognized rust hazard zones based on topography that are now used to formulate disease management systems in different areas.
Another epidemiological factor is the relative susceptibility of the different pine species and of different age classes of the same species. Of the three commercial species, sugar pine (Pinus lambertiana) is the most susceptible, western white pine (P. monticola) intermediate, and eastern white pine (P. strobus) least susceptible. Of all the white pines, whitebark pine (P. albicaulis) is most susceptible.
Young white pines, both saplings and pole size trees, are more severely infected and damaged than older trees for several reasons. On young trees the branches are closer to the ground, where conditions are more favorable for infection, and the needles are closer to the main trunk so that less time is needed for infections to get into the main stems and kill the trees. Mature trees can be infected, but these trees are not killed as rapidly and usually can be harvested before they die and deteriorate.
Factors important to development of wild Ribes include any soil disturbance, such as fire, logging, and snow and wind damage, which stimulates dormant Ribes seeds to germinate. Birds do not appear to be important in spreading Ribes seeds as they are for barberry, the alternate host of stem rust of wheat.
Disease Management
Quarantines and host removal
One of the earliest attempts to control blister rust was to destroy infected white pines as they were found, but the relatively long latent period of infection (the time from initial infection until symptoms or signs are produced) doomed this method. The next method attempted was to prevent further introduction and spread of the disease by laws (i.e., quarantines) that prohibited or restricted the movement of host plants from one region to another. The United States had no laws restricting the introduction of plants until the 1912 Plant Quarantine Act, which was a direct result of the introduction of blister rust. Quarantine No. 1 prohibited importing five-needled (i.e., white) pines into the country. A later quarantine, No. 26, prohibited taking currant and gooseberry bushes west of the Mississippi River. This was an attempt to restrict the disease to the eastern United States. But, as was later learned, the rust was already in the west, having been introduced on imported pines to Vancouver, British Columbia, in 1910. However, Quarantine No. 26 also authorized the destruction of cultivated currants, and this was accomplished in a relatively short time (Figure 10). The greatest effort at control was to destroy Ribes bushes from pine stands, but this method also failed. This attempt was based on the fact that spores from pine do not infect other pines, but that infection of pines came only from Ribes. Eradication of cultivated Ribes was relatively successful, but the abundance of wild Ribes spp. in remote and difficult terrain (Figure 11, Figure 12) made this impractical. Some eastern states still regulate the planting of susceptible, cultivated Ribes spp. (To find regulations of individual states, search for "ribes" at http://nationalplantboard.org/)

Figure 10 |

Figure 11 |

Figure 12 |
The eradication of wild Ribes was an extensive program and required much hand labor that often was not available. However, the blister rust control program got a tremendous boost when government relief programs during the Great Depression of 1933 provided work for the unemployed. The Civilian Conservation Corps was one of these programs and was designed to utilize labor on projects that would not compete with the private sector. These projects were primarily forestry and soil conservation. As many as 11,000 CCC men were employed in a single year for Ribes eradication in the national forests (Figure 13, Figure 14). In 1946, 175 people were employed for barberry eradication against wheat stem rust in the entire U.S. (see Stem rust of wheat lesson) vs. 2,500 people for Ribes eradication in the Northwest.

Figure 13 |

Figure 14 |
Rust hazard zones
High rust hazard zones occur where cool, moist air settles into depressions, such as valleys, stream bottoms and openings in forests, where air drainage is poor. In addition to creating favorable conditions for infection, air currents can carry spores for relatively long distances, i.e., several kilometers/miles, into these depressions. Generally, upper slopes and ridges are less conducive to blister rust infections than benches and valleys.
Pruning
Pruning to remove lower branches on young trees and removal of diseased branches (Figure 1) is recommended in some areas. Pruning of young trees should take place over several years, and no more than one-third of the branches should be removed at any one pruning. Removal of branches with cankers closer than 10-15 cm (4-6 in.) of the trunk is not feasible since the infection is probably already in the main stem. In addition to disease control benefits, pruning also promotes development of better quality wood for lumber. However, pruning is labor intensive and costly and requires a degree of judgment and skill.

Figure 1 |
Genetic resistance
The occurrence of apparently resistant trees in areas where the rust was severe (Figure 15) was recognized in eastern white pine as early as 1937. Surveys of heavily rusted sugar pine and western white pine stands led to selection of large numbers of apparently rust-free, and presumably resistant, trees as parent trees for disease resistance breeding programs. The programs for the western pines started about 1950. By crossing and backcrossing these parent trees and the subsequent progeny, a number of rust-resistant lines have been developed. These resistant trees are used to establish seed orchards and some are now producing seed for general distribution.

Figure 15 |
Several resistance mechanisms such as shedding of infected needles and slow canker development have been identified. Incorporating several different types of these mechanisms helps guard against the loss of resistance based on a single type.
Integrated management
At the present time, removal of highly susceptible Ribes spp., avoiding planting pines in high hazard zones, and planting resistant pines, if available, are recommended. In addition, pruning off lower branches and infected branches is suggested in some areas even though these measures are costly and require skilled workers. See Maloy, O. C. 2001. White pine blister rust. Online. Plant Health Progress doi:10.1094/PHP-2001-0924-01-HM for further information on management of white pine blister rust.
Historical Significance
The extensive white pine stands of northeastern, northwestern and Pacific Coast regions were among the most valuable timberlands in the United States. These pine species are among the most susceptible to blister rust. When large numbers of eastern white pine seedlings grown in European nurseries were imported to replant deforested areas, the rust came with them. The rust probably arrived in America about 1900, before there were any regulations restricting plant introductions, and was first discovered on currants in 1906 at Geneva, New York. It was found in 1909 on eastern white pine seedlings imported from European nurseries and on pines grown from local seed sources in 1915. Blister rust was introduced into the west on a single shipment of eastern white pine seedlings to British Columbia from France in 1910 but was not discovered until 1921. The rust spread rapidly and by 1950 was well established throughout the major white pine regions.
The white pines were valuable timber trees in the United States since the founding of the country. Before the American Revolution the British reserved all large white pine trees suitable for masts for exclusive use by the Royal Navy. In 1919, shortly after blister rust was discovered on native American white pines, the value of standing white pine timber was estimated to be over 1 billion dollars. Even today, a premium is still paid for logs of white pine over associated species. The value placed on white pines is reflected in the amount spent in trying to control the disease. An estimated $150 million was spent on the Ribes eradication program (Figure 13) and this does not include the resistance breeding programs In one peak year, 1949, over $6.5 million was appropriated for Ribes eradication. The 1955 U.S. Forest Service Timber Resources Review stated that of $3.8 million spent for direct control of forest diseases in 1952, $3.6 million (95 percent) was for blister rust control.

Figure 13 |

Figure 16 |
In some European countries and Canada, the fruit of currants and gooseberries has been valued more than the white pines. Figure 16 shows a collection of black, red, yellow and green currants and gooseberries grown for fruit (the large red berries on the middle right side are cranberries). These countries usually did not conduct Ribes eradication programs, but in recent years some have utilized disease resistant pines and cultural practices to combat the disease. Some rust-resistant gooseberries and red and black currants are now available from commercial nurseries.
There have been occasional reports that Pedicularis and Castilleja spp. are uredinial hosts of Cronarium ribicola and recent studies in the Northwestern United States support this. While the significance of this finding is still uncertain, if correct it certainly would have made ribes eradication futile, and it may be important in epidemiological and evolutionary aspects of white pine blister rust.
Selected References
Bingham, R.T. 1983. Blister rust resistant western white pine for the Inland Empire: the story of the first 25 years of the research and development program. USDA Forest Service Technical Report INT-146.
Boyce, J.S. 1961. Forest Pathology. 3rd ed. McGraw-Hill, New York, NY.
Fins, L., J. Byler, D. Ferguson, A. Harvey, M.F. Mahalovich, G. McDonald, D. Miller, J. Schwandt, and A. Zack. 2001. Return of the giants: Restoring White Pine Ecosystems by Breeding and Aggressive Planting of Blister Rust-Resistant White Pines. Univ. of Idaho Forest, Wildlife and Range Experiment Station Bulletin 72.
Hansen, E.M. and K.J. Lewis, eds. 1997. Compendium of Conifer Diseases. American Phytopathological Society, St. Paul, MN.
Kinloch, B.B., Jr., R. A. Sciezko, G. D. Barnes, and T. E. Greathouse. 1999. A major gene for resistance to white pine blister rust in western white pine from the Western Cascade Range. Phytopathology 89: 861- 867.
Maloy, O.C. 1997. White pine blister rust control in North America: A case history. Annual Review of Phytopathology 35:87-109.
Maloy, O. C. 2001. White pine blister rust. Online. Plant Health Progress doi:10.1094/PHP-2001-0924-01-HM.
McDonald, G.I., B.A. Richardson, P.J. Zambino, N.B. Klopfenstein, and M.-S. Kim. 2006. Pedicularis and Castilleja spp. are natural hosts of Cronarium ribicola in North America: a first report. Forest Pathology 36: 73-82.
