This disease lesson has been revised. Click here to view the current version.
Davis, E.L. and G.L. Tylka. 2000. Soybean cyst nematode disease. The Plant Health Instructor. DOI: 10.1094/PHI-I-2000-0725-01
Updated 2005 and 2021.
Soybean cyst nematode disease
Heterodera glycines
Soybean (primary economic host), other legumes
Authors
Eric L. Davis, North Carolina State University, Raleigh
Gregory L. Tylka, Iowa State University, Ames

Soybean field with large area of obvious soybean cyst nematode damage |
Microscopic worms burrowing through cells, injecting foreign compounds through hypodermic-needle-like structures, altering the basic biology of the cells being fed upon. Worms that grow so large in just a few weeks that they rupture out of the tissue they are feeding upon. Sound like a science fiction movie plot? Believe it or not, it is the basic biology of a widespread, serious pathogen of soybeans - the soybean cyst nematode.
Symptoms and Signs
High population densities of the soybean cyst nematode (SCN) can result in large portions of soybean fields with plants that are severely stunted and yellow (Figures 1 and 2). More frequently, however, few aboveground symptoms of SCN can be observed - yet soybean yield losses of 10-20% or more can be attributed to SCN damage in these fields (Figure 3). This hidden cause of yield loss has presented a major problem for growers who must learn to recognize when their fields are infested with SCN. Reduced yields in SCN-infested fields often are attributed to adverse cultural or environmental conditions rather than to SCN - a tragic mistake that leads to increased SCN infestation and damage in subsequent years. Also, the age and vigor of the soybean plants, the nematode population density in the soil, soil fertility and moisture levels, and other environmental conditions influence the intensity of the symptoms. Soybean cyst nematode damage usually is more severe in light, sandy soils, but will occur readily in all types of soil.

Figure 1 |

Figure 2 |

Figure 3 |
The whitish-yellow adult females of SCN on the outside of roots dug from the field are a telltale sign of SCN infestation that can be easily seen (Figure 4). The SCN females are lemon-shaped, slightly less than 1 mm in diameter, and considerably smaller than Bradyrhizobium root nodules (Figure 5). Reduced nodulation was associated with infection of soybean roots by populations of SCN identified as race 1. Analysis of soil and root samples by a diagnostic lab, however, is the only reliable means to confirm SCN diagnosis. The vermiform (worm-shaped) juvenile and adult males can be extracted from soil samples, as well as the brown cysts (dead female body containing eggs) and freed eggs in the soil. Developing SCN juveniles and females can be observed in stained soybean root samples. Diagnostic labs also can estimate the population density of SCN in a field to provide recommendations of SCN management options. Determination of the race of SCN in a field is a laborious task, and it may or may not be helpful in choosing a soybean cultivar for that site.

Figure 4 |
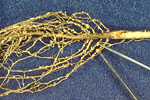
Figure 5 |
Pathogen Biology
Early reports of the cyst-forming nematodes on soybeans from Asia classified them as variants of the sugar beet cyst nematode, Heterodera schachtii, and occasionally as the pea cyst nematode, Heterodera goettingiana. Ichinohe described the soybean cyst nematode (SCN) as a distinct species, H. glycines, in 1952. Morphological features of the adults of SCN are used for species identification. SCN adults are sexually dimorphic, meaning that they are dissimilar in appearance. The females are swollen and sedentary, and the males are vermiform (worm-shaped) and motile. Only the swollen female stage on the surface of soybean roots can be seen with the naked eye – the male and juvenile stages must be extracted from soil or plant roots and viewed under a microscope.
The second-stage juveniles (J2) of SCN are worm-shaped, 375-520 µm long, and about 18 µm in diameter. The overall body shape of the nematode is determined by the pressure of its internal body fluids pushing against its strong, but flexible, outer "cuticle" (like a water balloon). The outside of the cuticle has a series of fine rings (annulations), like an accordion, that allows the cuticle to bend at any point along the nematode's body. The cuticle is composed mainly of the structural protein collagen, and the cuticle is molted four times to allow growth and maturation of the nematode. The "head" of the nematode can be recognized by the presence of a short, dark spear with basal knobs (the "stylet") just inside the tip of the head (Figure 6). The stylet is hollow (like a hypodermic needle) and protrudes from the head when used by the nematode for feeding from plant cells and penetrating plant tissues. The very outer tip of the nematode head above the stylet (called the "lip" region) is slightly elevated, rounded, and darkened in J2 of SCN. In a relatively clear area just below the stylet, a round, muscular pumping organ called the metacorpus can be seen - the metacorpus pumps substances (i.e., food and secretions) up and down the esophagus of the nematode. Just below the metacorpus is another relatively translucent area that contains three esophageal glands that overlap the nematode's intestine on the ventral (stomach) side of its body. The intestine can be recognized as a fairly long, dark area extending from the esophageal glands to the tail of the nematode. The tail of SCN J2 tapers uniformly to a fine, rounded tip that is hyaline (clear).
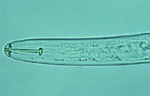
Figure 6 |
Adult females of SCN are the most easily diagnosed stage for this species. The females are lemon-shaped, ranging from 500 to 900 µm long and 200 to 700 µm wide (Figure 7a). The female's stylet is slender with knobs that project slightly backward. The vulva and anus collectively form a cone-shaped projection from the rear of the female's body, giving them a characteristic "lemon" shape. The SCN females swell and molt through the juvenile life stages until they protrude from the plant roots (see Disease Cycle). The adult females of SCN are initially white (on the root surface), and their heads are buried within the root for feeding. As the female ages, she turns yellow and eventually brown as she dies. Her body becomes a protective cyst encasing the eggs.

Figure 7a |
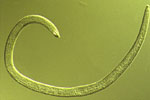
Figure 7b |
Adult males of SCN are worm-shaped and relatively long (Figure 7b), compared to second-stage juveniles. They are 1,200 to 1,400 µm long and 26 to 30 µm wide. Although the head, stylet, and esophagus are similar to second-stage juveniles, the tail is short with a blunt, rounded terminus. An easy, defining characteristic of males is the presence of two dark hooks (called "spicules") that are always present at the opening of the testis near the tail.
Disease Cycle and Epidemiology
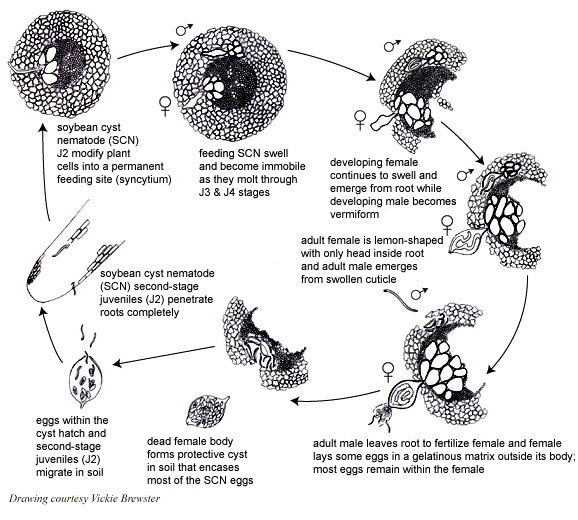
Like all nematodes, the soybean cyst nematode (SCN) has six life stages - egg, four juvenile stages (J1-J4), and the adult stage. The duration of the SCN life cycle runs from 3-4 weeks, but this may be influenced by environmental conditions (mainly adequate temperature and moisture). Depending upon the environment, several generations of SCN can be completed in a typical soybean growing season. After embryonic development within the egg to the first-stage juvenile, the nematode goes through four molts to the adult stage. The molt to second-stage juvenile (J2) occurs within the eggshell (Figure 8), and it is the J2 that emerges from the egg. Egg hatch seems to have evolved as a survival strategy in SCN. A low percentage of SCN eggs appear to hatch spontaneously -- it has been suggested that these eggs are the ones that are laid in a gelatinous matrix outside the female body. A significant proportion of eggs that are retained within cysts are in a dormant state -- they do not hatch until soybeans are planted for the next growing season. It is hypothesized that exudates from soybean roots provide the hatching signals for dormant SCN. Another proportion of eggs within cysts do not hatch, even when conditions are favorable - they are in a state termed diapause. Diapause appears to be a time-mediated hatching process, the basis of which is not presently understood.
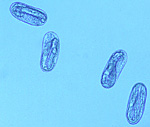
Figure 8 |
The J2 is the infective stage of SCN. The J2 migrates in soil and penetrates plant roots completely, usually just behind the root tip (Figure 9). The J2 moves intracellularly to the root vascular tissue, often leaving a zone of visible root necrosis along their migratory path within the root. The J2 uses thrusts of its stylet and secretes cell-wall-degrading enzymes (cellulases) to migrate directly through plant cells (Figure 10). When the J2 reaches the root vascular tissue, nematode stylet secretions modify selected plant cells into an elaborate feeding site called a syncytium (Figure 11). The J2 will not continue development unless a syncytium is formed for feeding. The syncytium is a large, metabolically active feeding site that becomes multinucleate as neighboring plant cells are incorporated into the syncytium by cell wall dissolution and cell fusion. The J2 feeds from the syncytium and begins to swell and become immobile (Figure 12).
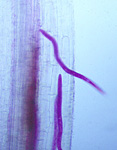
Figure 9 |
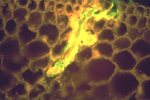
Figure 10 |
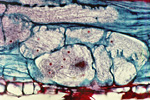
Figure 11 |

Figure 12 |
The subsequent juvenile stages molt and continue to enlarge as the nematode feeds. Approximately half of the juveniles will become swollen females, and the majority of the female body (except for the head) breaks through the root surface and becomes visible on the surface of the root (Figures 13, 14). Males develop coiled within the swollen J4 cuticle (Figure 15), and they emerge from the cuticle and root as motile, vermiform adult nematodes (Figure 7).
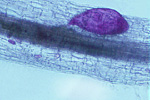
Figure 13 |

Figure 14 |
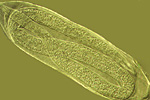
Figure 15 |
It has been documented that a higher percentage of males is produced when the nematodes or host plants are under stress. Males do not feed, but they are required for sexual reproduction (copulation) with females that are exposed on the root surface. After fertilization, the majority of the 200-600 eggs produced by the female are retained within its body, but some eggs may be laid in a gelatinous matrix extruded from the posterior (vulva) of the female. As the gravid female dies, its cuticle becomes a brown, hardened structure (the cyst) that encases and protects hundreds of viable eggs (Figure 16). Cysts often fall from roots and remain free in the soil.

Figure 16 |
Epidemiology
As with many plant-parasitic nematodes in soil, soybean cyst nematodes do not move far from the root zone that they currently infest. In most cases, the natural migration of SCN within a field is defined as "contagious" – small patches of infested areas that gradually enlarge to encompass significant areas of disease. Diseased areas become much more pronounced in sections of soybean fields that are under environmental stress (i.e., insufficient fertilization or water, extreme temperatures). The spread of these nematodes within fields usually is accelerated by cultural practices of the grower (i.e., dissemination of nematodes by soil cultivation). SCN can be introduced to uninfested sites via poorly sanitized farm equipment and within soil peds (small, seed-size clumps of dried soil) within contaminated seed stocks (Figure 17). SCN is not transmitted inside individual seeds nor on the seed surface (SCN is not "seed borne"). The cysts of SCN are lightweight and can survive desiccation. They can be transmitted in surface water, by wind, and also by the movement of animals. It can take from 5-9 years from the time of introduction of SCN into a field until the SCN populations reach detectable levels. By that time, infestation with SCN has become a permanent problem in that site.

Figure 17 |
Disease Management
Once established in a field, SCN cannot be eradicated. However, there are various practices that can be implemented in an integrated pest management (IPM) program to minimize SCN reproduction and maximize soybean yields in infested fields.
Scout for early detection (look for symptoms and signs in the field). Aboveground symptoms can range from nonexistent to severe, and they are influenced by many factors. (One cannot rely solely upon aboveground symptoms, however, for definitive identification of SCN infestations). If the soybean yield obtained in a particular field has leveled-off or decreased for no apparent reason, or if SCN has been confirmed on nearby land, it is recommended that soil samples be taken from the plant root zone (Figure 18). A professional diagnostic lab should examine the samples for the presence of SCN. Soybean roots should also be examined directly for the presence of SCN females on the root surface (Figures 4 and 5).

Figure 18 |
Genetic resistance
Grow SCN-resistant soybean cultivars. Resistant soybean cultivars are an effective management tool (Figure 19). Planting resistant soybeans in SCN-infested fields will usually reduce reproduction of the nematode. Most SCN juveniles do not feed and hence are unable to complete their life cycle on the roots of resistant cultivars; a few, however, will survive and reproduce. Some resistant soybean cultivars may yield slightly less than susceptible cultivars in noninfested fields, but they will yield significantly better than susceptible cultivars in fields infested with SCN. If the same resistant cultivar is grown in the same field year after year, SCN has the ability to adapt to the cultivar, or “break resistance.” To reduce the possibility of this happening, some university researchers recommend that growers alternate use of the soybean cultivars with different sources of SCN resistance, and also that a susceptible cultivar be grown once after all types of available resistance have been rotated. The “HG-Type” system that has replaced the race system indicates which genetic sources of soybean resistance any given population of SCN can infect.

Figure 19 |
Cultural practices
Grow SCN nonhost crops. SCN is an obligate parasite; the nematode is unable to mature and reproduce in the absence of host roots. Consequently, SCN population densities decline during any year that nonhost crops are grown. For example, a 1-2 year rotation with corn (nonhost) has proven effective for many growers (Figure 20). The magnitude of decline of SCN population densities during a year that a nonhost crop is grown is somewhat unpredictable, however, because the change in population density varies from year to year and is greatly influenced by environmental conditions. SCN-resistant soybean cultivars often are incorporated into a multi-year cycle of rotations with nonhosts crops – this combination of practices is an excellent integrated management strategy.

Figure 20 |
Maintain a healthy crop. Plants that have adequate moisture and nutrients are better able to withstand infection by SCN. In land infested with SCN, maintaining proper soil fertility and pH levels and minimizing other plant diseases, insect, and weed pests that weaken the plants is more critical to maximizing soybean yield than when land is noninfested.
Manage the movement of soil. If only certain fields on a farm are infested, planting and cultivating of infested land should be done only after noninfested fields have been worked. Soil on equipment should be thoroughly removed with high-pressure water or steam, if available, after working in infested fields (Figure 21). Also, seed grown on infested land should not be planted in noninfested fields unless the seed has been properly cleaned; SCN may be spread in the seed-size soil peds (clumps) mixed in with the seed (Figure 17).

Figure 21 |
Chemical control
Use nematicides. No nematicide will kill all SCN in the soil. There are a few nematicides that are labeled for use against SCN, including the fumigant 1,3-dichloropropene (Telone) and the nonfumigants aldicarb (Temik or Bolster) and oxamyl (Vydate). Different products may be labeled in different states. When applied at planting, the effect of the nematicides may last long enough to provide an economic yield benefit. By the end of the growing season, however, SCN numbers may be as high or higher than they were at planting. The performance of the nematicide will depend on soil conditions, temperatures, and rainfall. Yield and economic benefits are not guaranteed, and nematicides are expensive. All nematicides are extremely toxic, especially the nonfumigants like Temik and Vydate that are nerve poisons. Only licensed applicators may use these pesticides.
Significance
The first documented report of damage by the soybean cyst nematode (Heterodera glycines Ichinohe) was by S. Hori in Japan in 1915. Ancient Chinese literature and personal communications, however, suggest that SCN may have been a pathogen of soybean in China as early as 235 B.C. The term "soybean yellow dwarf" was adopted by Japanese researchers in the 1920s to describe the pale yellow areas of poor soybean growth observed in fields.
SCN was first reported in the United States in 1954 in Hanover County, North Carolina – an area known to import flower bulbs from Japan. The rapid spread of SCN to other soybean-growing states has prompted some hypotheses that the nematode is indigenous and parasitizing some leguminous weeds in the U.S. However, there are indications that soybean seed had been imported to the United States as early as 1765. More significant may be the importation of soil from Asia in the late 1800's to obtain inoculum of Bradyrhizobium japonicum for nitrogen fixation in soybean. Some imported soil was distributed among researchers who were working on the improvement of soybean cultivation in the U.S. Soil also was shared among growers to obtain nitrogen-fixing inoculum once its utility had been demonstrated.
The rapid expansion in production acreage of soybean in the U.S. that started in the mid-1900s served to establish a huge reservoir of host crop for the increase of SCN. A federal quarantine for SCN was established in 1957, but the quarantine was lifted in 1972 because it was ineffective. It has been suggested that the movement of SCN-infested soil and plant material into new soybean production areas had already occurred before the quarantine could be established. SCN now infests every soybean-producing state in the U.S. (Figure 22), with total soybean yield loss estimates approaching $1 billion per year! Though SCN has been a recognized pathogen of soybean in the southeastern United States since the 1950s, the detection and effects of SCN in the midwestern U.S. "soybean belt" has been more recently realized.
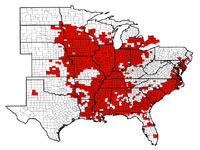
Figure 22 |
SCN was detected in Colombia, South America, in the early 1980s, and was soon thereafter found in Argentina and Brazil - two of the world's important soybean production areas. More recently, SCN was reported from Italy, and if soybean production is expanded in Europe, more SCN infestations will likely be found. In a recent survey of the top ten soybean- producing countries in the world, SCN was found to be the most damaging pathogen of soybean.
Selected References
Baldwin, J.G., and M. Mundo-Ocampo. 1991. Heteroderinae, cyst and non-cyst-forming nematodes. Pages 275-315 in Manual of Agricultural Nematology. Nickle, W.R., ed. Marcel Dekker, Inc., New York
Barker, K.R., G.A. Pederson and G.L. Windham. 1998. Plant and Nematode Interactions. ASA, CSSA, SSA Publishers, Madison, WI.
Davis , E.L. and M.G. Mitchum. 2005. Nematodes: sophisticated parasites of legumes. Plant Physiology 137:1182-1188.
Evans, K., D. L. Trudgill and J. M.Webster, eds. 1993. Plant Parasitic Nematodes in Temperate Agriculture. CAB International, Cambridge, UK
Hartman, G.L., J.B. Sinclair and J.C. Rupe. 1999. Compendium of Soybean Diseases, fourth edition. American Phytopathological Society, St. Paul, MN.
Niblack, T. L., P. R. Arelli, G. R. Noel, C. H. Opperman, J. H. Orf, D. P. Schmitt, J. G. Shannon, and G. L. Tylka. 2002. A revised classification scheme for genetically diverse populations of Heterodera glycines. Journal of Nematology 34:279-288.
Schmitt, D. P., J. A. Wrather, and R.D.Riggs (eds.) 2004. Biology and Management of the Soybean Cyst Nematode (Second Edition). Schmitt & Associates of Marceline, Marceline, MO.
Wrather, J.A., T.R. Anderson, D.M. Arsyad, Y. Tan, Y.J. Gai, L.D. Ploper, A. Porta-Puglia, H.H. Ram and J.T. Yorinori. 2001. Soybean disease loss estimates for the top 10 soybean-producing countries in 1998. Canadian Journal of Plant Pathology 23:115-121.
Wyss, U., and U. Zunke. 1992. Observations on the feeding behavior of Heterodera schachtii throughout development, including events during molting. Fundamental and Applied Nematology 15:75-89.
