Thomas, S.L., L.H. Rhodes, and M.J. Boehm. 2004. Following the disease progression of an ectotrophic root-infecting fungus.The Plant Health Instructor. DOI: 10.1094/PHI-I-2004-1215-01
Soilborne root-infecting fungi can be damaging to susceptible hosts. Control of diseases caused by root-infecting fungi is difficult when symptom expression within the host is delayed until periods of unfavorable environmental conditions, such as heat or drought stress. By the time symptoms develop, up to 50% or more of the host plant's roots may be infected. Some disease examples caused by root-infecting fungi include take-all of wheat, Pythium root rot, Rhizoctonia root rot of beans, and black root rot of pansies (caused by
Thielaviopsis basicola).
Gaeumannomyces graminis is an ascomycetous fungus that infects and colonizes roots of graminaceous plants. Three morphological variants of this fungus have been described, but all three varieties produce specialized
appressoria, called
hyphopodia, and darkly-pigmented,
ectotrophic, runner hyphae . Hyphopodia function as organs which attach the fungus to host roots. Darkly-pigmented, ectotrophic, runner hyphae grow along the root exterior and disseminate the pathogen by growing from root to root.
Take-all of wheat (Triticum aestivum), caused by
Gaeumannomyces graminis var.
tritici, is a devastating disease on wheat grown in the Pacific Northwest region of the United States. The disease cycle of the fungus is fairly simple as the fungus survives, disseminates, and infects through mycelial growth (Figure 1). The fungus may survive between wheat crops in infested wheat residues or on the roots of weedy grass species that may grow in or near wheat fields. When a new wheat crop is planted, the fungus grows out from infested crop or weed residue and infects newly seeded plants (Figure 2). The fungus will grow ectotrophically producing hyphopodia and infection hyphae which then penetrate the root and begin the colonization process. Fungal mycelium grows throughout the host roots, around plant cells, and eventually into the stele. Once
G. graminis var.
tritici begins colonizing the stele, vascular dysfunction occurs, and water and nutrient uptake from the soil is reduced. Vascular discoloration (Figure 3), a key diagnostic symptom, is produced by fungal colonization of the stele. Fungal spread from plant to plant occurs as dark ectotrophic, runner hyphae grow from root to root. Once a host is colonized,
perithecia are typically produced on the plant crown or embedded in host tissues (Figures 4, 5). Perithecia contain many
asci (Figure 6) and each ascus contains eight
ascospores (Figure 7). Ascospores are not considered to play an important role in disseminating this pathogen.
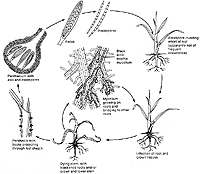
Figure 1 |

Figure 2 |
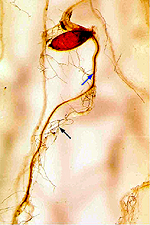
Figure 3 |
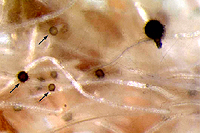
Figure 4 |

Figure 5 |
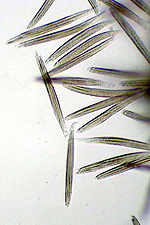
Figure 6 |
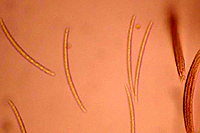
Fig |
Similar disease cycles occur for other
G.
graminis-incited diseases, such as those caused by
G.
graminis var.
avenae on creeping bentgrass (Agrostis palustris) and oats (Avena sativa) and
G.
graminis var.
graminis on rice (Oryza
sativa). The objective of this exercise is to familiarize students with the root-infection process utilizing test tube assays which permit the observation of the disease progression. This assay is also a good way to produce perithecia of
G. graminis. (Laboratory Instructor's Note #1)
CLICK HERE FOR INSTRUCTOR'S NOTES
For Instructor/Laboratory preparers: (Laboratory Instructor's Note #2)
- 10% bleach solution (Final concentration ~0.525% NaOCl) containing 0.1% Tween 20 (400 ml)
- Distilled water
- Wheat seeds (200 seeds per 50 students) (Laboratory Instructor's Note #2)
- Whatman no. 1 filter paper (4-10 cm discs) or 2 paper towels
- Self closing plastic bag or Petri dishes
- Agar
- Disposable 25-ml pipets and pipet bulb
- Creeping bentgrass cv. Penneagle seed (10 ml)
- Cultures of
Gaeumannomyces graminis var.
avenae (Lab Instructor's Note # 3)
- No. 3 cork borer (8 mm dia
- m.)
- 16 X 100 mm borosilicate test tubes (pre-sterilized); 4 per student
Preparation of materials for students (by instructor):
-
Preparation of cultures. Cultures of
G. graminis var.
avenae should be transferred to fresh 1/5 strength potato dextrose agar 7-10 days prior to use. This ensures the plates are completely colonized and actively growing. Using good aseptic technique, cut agar disks into the media using a no. 3 cork borer. Two plugs will be needed per student. Agar disks can be cut into the agar up one day in advance of the lab.
-
Pre-germination of wheat seeds. Surface-sterilize wheat seeds in 200 ml of the 10% bleach solution for 5 min, then thoroughly rinse with 300 ml of distilled water three times. Place wheat seeds on moistened filter paper or paper towels and spread out. Place paper and seeds in a plastic bag, seal closed with a little air in the bag, and incubate for 3-4 days at room temperature under natural light (Lab Instructor's Note #4, 5).
-
Preparation of agar tubes. Prepare 1 liter of 0.3% water agar (3 g agar per liter) per 45 tubes to be used. Autoclave for 20 min then dispense 20 ml into each test tube. Allow tubes to cool in an upright position for a minimum of 2 hours prior to use. Tubes can be covered with parafilm or sterile test tube caps and stored at room temperature for 3-4 days before use.
-
Preparation of creeping bentgrass seed. An hour before lab, surface sterilize the creeping bentgrass seed following step 2. Place rinsed seed in a petri dish on a piece of filter paper to dry. Shake the dish gently to break up any clumps of seeds.
For Students:
- 4 test tubes containing 0.3% water agar
- A culture of
G. graminis var.
avenae with agar discs pre-
- cut
- Sterilized toothpicks or dissecting probe
- Alcohol lamp or gas burner (if using a dissecting
- probe)
- Sterilized wooden applicator sticks
- Pre-germinated wheat seeds
- Surface-sterilized creeping bentgrass seed
- Forceps
- Parafilm
- Test tube rack
-
Labeling of test tubes. Label test tubes with the following information: name, date, fungal isolate, lab section and host (two for creeping bentgrass, two for wheat). For each pair of test tubes for each host, label one tube as control and one as inoculated.
-
Preparation of 2 control
- tubes.
- Into one test tube, sprinkle a pinch of the sterilized creeping bentgrass seed. Seal the tube with parafilm and place into the test tube rack.
- Surface-sterilize forcep tips over an alcohol flame for a few seconds. Allow the forceps to cool for a few seconds, then gently pick up a pre-germinated wheat seed and place it roots-down onto the top of the water agar layer in the test tube. Seal the tube with parafilm and place in the test tube rack.
-
Preparation of 2 inoculated tubes.
- If using a probe, sterilize the tip over an alcohol burner and cool for a few seconds before using. With the tip of the probe or a sterile toothpick, gently lift an agar disk from the colonized culture plate and drop it onto the water agar. Gently press the agar disk with a wooden stick to submerge it about 2.5 cm (1 in.) into the water agar.
- Repeat the steps of 3a with the second tube.
- Seed the tubes following Steps 2a and 2b for the 2 inoculated tubes.
-
Incubation of test tubes. Test tube racks with tubes should be placed in a growth chamber set to the following conditions: 15oC; 18 hr photoperiod. Alternatively, the racks can be incubated in a windowsill with natural light. (Laboratory Instructor's Note #6)
-
Continued growth of wheat. Once wheat seedlings have produced leaves longer than the tube, remove parafilm and plug the opening with a cotton plug permitting the wheat leaves to grow out of the tube. (Laboratory Instructor's Note #7)
-
Continued growth of creeping bentgrass. The creeping bentgrass seed should germinate in 7-10 days. Remove parafilm 14 days after assay set-up from the bentgrass tubes. (Laboratory Instructor's Note #8)
-
Observation of disease progress. Observe plant development and root colonization weekly under a dissecting microscope through the tubes and note the disease progression in Table 1. Be sure to note the following: mycelial growth from the agar plug, initial infection points, colonization of the host, and symptom expression.
-
Formation of perithecia. Seal the creeping bentgrass tubes with parafilm at 28 days to maintain moisture for perithecial development.
-
Observations of perithecia. Obtain a pair of forceps, alcohol lamp or gas burner, a microscope slide, a cover slip and water dropper bottle. Surface sterilize the forceps tips over a flame and cool for a few seconds. Remove the parafilm or cotton plug from the inoculated tube, and using the forceps, gently retrieve bentgrass or wheat plants with perithecia. Place the plant tissue onto the microscope slide and add a drop of water. Add the cover slip and observe the perithecia under a compound microscope. If asci and ascospores are not ejected, a little pressure can be applied to the cover slip to break open perithecia.
Table 1. Be sure to note pathogen and host growth, the date root infection is first observed on each host, and any signs or symptoms as they are produced. (Laboratory Instructor's Note #9)
- Describe two examples of how aseptic technique was used in this experiment. Why do you think this was important?
- Were there differences in the rate of root infection and perithecial development between creeping bentgrass and wheat?
- What was the purpose of including non-inoculated tubes? Were there any differences observed between the non-inoculated and the inoculated tubes? If so, what were they?
- Describe where the perithecia were produced on each host. Given their location, what role do you think perithecia and ascospores could play in the disease cycle?
- From your observations, how do you think the fungus is affecting the water uptake of the plants?
LITERATURE CITED:
Bockus, W.W. and N.A. Tisserat. 2000. Take-all root rot. The Plant Health Instructor. DOI:10.1094/PHI-I-2000-1020-01.
Clarke, B.B. and A.B.Gould, eds. 1993. Turfgrass Patch Diseases Caused by Ectotrophic Root-infecting Fungi. American Phytopathological Society Press, St. Paul, MN.
Smiley, R.W., P.H. Dernoeden, and B.B. Clarke. 1992. Compendium of Turfgrass Diseases, 2nd Ed., American Phytopathological Society Press, St. Paul, MN.
