Updated 2006.
OBJECTIVES:
To become familiar with the cytological events involved in the establishment of infection by a fungal pathogen.
To understand the effect of various management practices on particular infection events, and the significance of this to disease management.
CLICK HERE FOR INSTRUCTOR'S NOTE 1
|
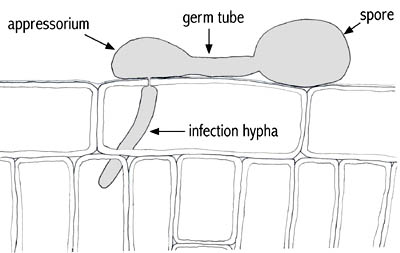
Figure 1. Schematic representation of the fungal structures present during infection. |
Figure 1 provides a diagrammatic representation of important structures formed during the infection process of many fungi. Those you will see in this laboratory are:
-the
germ tube, a slim fungal tube emerging from the spore upon germination
-the
appressorium, a swollen structure at the end of a germ tube which attaches to the plant cell about to be penetrated
-infection hypha. For the purposes of this lab, all fungal structures within host cells can be considered as "infection hyphae."
Prepare for laboratory by reviewing the items below in Agrios (1) or other general plant pathology textbooks.
Be sure to prepare for your portion of the discussion session, as outlined in Table 3.
1.
Fungicides. Learn the difference between a contact (protectant) fungicide and a systemic fungicide. Although a glossary may help, the index in your textbook may guide you to good descriptions in the text.
2.
Biological control. Learn that biocontrol agents often interfere with pathogen activity by one or more of the following mechanisms: (1) parasitism of the pathogen, (2) competition with the pathogen for nutrients or other resources, (3) excretion of toxic substances or (4) induction of host resistance. For students with access to Agrios (1), read about "Mechanism of Action" on p. 307 and view the images in Figures 9-7 through 9-13.
3.
Host resistance. Learn what
phytoalexins are (just a general working definition, not specifics about particular phytoalexins); the glossary and index of your text may serve as a guide to appropriate information. Although plants produce many types of
biochemical defense mechanisms, phytoalexins will serve as a good example. Also learn that plants sometimes defend themselves with
structural defense mechanisms produced in response to infection. For students with Agrios(1), examine Figure 6-6, which illustrates formation of a
cork layer in a potato tuber, as an example of a structural defense mechanism.
MATERIALS
1. A suspension of
conidia (a type of asexual spore) of
Pyricularia oryzae. Strains of this fungus cause rice blast, gray leaf spot of perennial ryegrass, and other diseases of monocots.
2. Prepared slides of leaves of perennial ryegrass which were inoculated with conidia of
P. oryzae and incubated for various times periods. These were prepared by removing leaves from 3-wk old perennial ryegrass plants; clearing the tissue by boiling for 1-2 min in a 1:1 mixture of glacial acetic acid and 95% ethanol followed by rinsing in sterile distilled water; and placing leaves on clean glass slides. These were sprayed with a suspension of 5 ×104 conidia/ml and incubated at room temperature at 100% relative humidity for various times. Fungal structures were stained by wicking the water from the spore suspension off the leaves, adding 1% trypan blue in lactophenol, placing a cover slip, and heating for several seconds over an alcohol lamp (while trying to avoid boiling the sample).
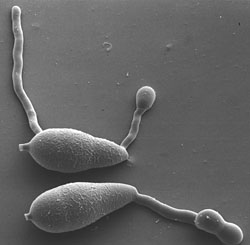
|
Figure 2. Conidia of
P. oryzae with germ tubes and appressoria (image provided by R. J. Howard, first published in Howard, R.J. Cell biology of pathogenesis. pp. 3-22 In: R.S. Zeigler, S. Leong, and P.S. Teng, eds.
Rice Blast Disease. CAB International. Oxon, United Kingdom; reproduced with permission). |
CLICK HERE FOR INSTRUCTOR'S NOTE 2
PROCEDURE
1. Familiarize yourself with the morphology of conidia of
P. oryzae by placing a droplet of a fresh spore suspension on a glass slide, placing a cover slip, and viewing at 40-400×magnification. Select ten conidia at random, and determine and record the most advanced cytological stage for each in Table 1.
2. Choose a prepared slide incubated for 1 hr. Examine the slide at 40-400×magnification. First, take the time necessary to familiarize yourself with the various fungal structures (germ tubes, appressoria, infection hyphae) present in your mount. Then select ten conidia at random, and determine and record in Table 1 the most advanced cytological stage present for each.
3. Repeat this process with a slide incubated for 2 hr, 7-8 hr, 14-16 hr, and 24 hr, or as assigned by your instructor.
CLICK HERE FOR INSTRUCTOR'S NOTE 3
4. Provide your results to the class by filling in a column in the table on the blackboard. When all individuals lab groups have provided their data, insert those totals into Table 2.
OBSERVATIONS
[LAB SHEETS TO BE PRINTED AND FILLED OUT BY STUDENTS]
Table 1. Data Sheet for Individuals or Lab Groups
Number of conidia at cytological stage indicated |
Incubation
Period (hr) | Ungerminated | With germ
tube only | Immature
appressoriuma | Mature appressoriumb | Infection
hypha |
| 0 | | | | | |
| 1 | | | | | |
| 2 | | | | | |
| 7-8 | | | | | |
| 14-16 | | | | | |
| 24 | | | | | |
a stained dark blue
b stained light blue, has a brown tint from melanin accumulation in the cell wall of the appressorium. |
Table 2. Data Sheet Summarizing Lab Section Results
Number of conidia at cytological stage indicated |
Incubation
Period (hr) | Ungerminated | With germ
tube only | Immature
appressoriuma | Mature appressoriumb | Infection
hypha |
| 0 | | | | | |
| 1 | | | | | |
| 2 | | | | | |
| 7-8 | | | | | |
| 14-16 | | | | | |
| 24 | | | | | |
a stained dark blue
b stained light blue, has a brown tint from melanin accumulation in the cell wall of the appressorium. |
Table 3. Discussion Questions and Assignments
Question | Name of student(s) responsible for providing an initial answer |
| 1. At which cytological stage(s) do contact fungicides act? | |
| 2. At which cytological stage(s) do systemic fungicides act? | |
| 3. At which cytological stage(s) do phytoalexins act? | |
| 4. At which cytological stage(s) does cork formation act? | |
| 5. At which cytological stage(s) is a biocontrol agent most likely to interact with the pathogen? | |
| 6. At which cytological stages(s) is the pathogen most sensitive to prolonged exposure to dry air? | |
| 7. At which cytological stage(s), if any, will symptoms be present? | |
Conclusions and Questions
We will conclude the lab with a discussion on the significance of the various cytological events in the infection process. The questions in Table 3 will be covered. Come to class prepared so that you can participate in the discussion when prompted.
CLICK HERE FOR INSTRUCTOR'S NOTE 4
When you leave lab, you should feel competent to answer the following questions, as well as those in Table 3.
1. Compare and contrast the cytological stages at which contact fungicides and systemic fungicides act. What is the significance of this to disease management?
2. Many important host defense mechanisms against pathogens act at the same cytological stage of the disease cycle as do phytoalexins and cork formation. Thus, at which stage do many important host defense mechanisms act?
3. At which cytological stage is
Pyricularia oryzae most sensitive to environmental extremes?
4. You are inspecting leaves showing symptoms of spotting and blighting due to a leaf-infecting fungus. Which fungal cytological events and events in the disease cycle (SEE STUDENT NOTES BELOW) have occurred at this point? Will application of a contact fungicide at this point eradicate these infections? What about a systemic fungicide?
Get the Answers
STUDENT NOTES
Events common to disease cycles are:
- Pathogen survival
- Production of primary inoculum
- Dispersal of primary inoculum
- Infection
- Host colonization
- Symptom development
- Production of secondary inoculum (for polycyclic pathogens)
- Dispersal of secondary inoculum (for polycyclic pathogens)
LITERATURE CITED
1. Agrios, G. 2005. Plant Pathology, 5th edition. Elsevier Academic Press, Burlington, MA.
