Mathew, F., Harveson, R., Gulya, T., Thompson, S., Block, C., and Markell, S. 2018. Phomopsis Stem Canker of Sunflower. Plant Health Instructor. https://doi.org/10.1094/PHI-I-2018-1103-01
View Spanish Version
Phomopsis stem canker of sunflower
Diaporthe helianthi Muntañola-Cvetkovic, Mihaljcevic and Petrov (syn. Phomopsis helianthi), Diaporthe gulyae Shivas, Thompson and Young, several species of
Diaporthe (syn. Phomopsis)
Sunflower (Helianthus annuus)
Authors
Febina Mathew, South Dakota State University, Brookings, SD, USA
Robert Harveson, University of Nebraska-Lincoln, Scottsbluff, NE, USA
Thomas Gulya, USDA-ARS Northern Crop Science Laboratory, Sunflower and Plant Biology Research Unit, Fargo, ND, USA (Retired)
Susan Thompson, University of Southern Queensland, Toowoomba, QLD, Australia (Retired)
Charles Block, Iowa State University, Ames, IA, USA
Samuel Markell, North Dakota State University, Fargo, ND, USA
 |
Figure 1- Leaf infection from Phomopsis stem canker. |
Phomopsis stem canker is a major yield limiting disease of sunflower (Helianthus annuus L.) in the world (Harveson et al. 2016). The disease was first described in 1980 from the Vojvodina region of the former Yugoslavia and the causal fungus was
Diaporthe helianthi (syn.
Phomopsis helianthi) (Muntañola-Cvetkovic et al. 1985). Following this first disease report, Phomopsis stem canker was reported in the 1980s and 1990s from several sunflower producing countries including Hungary (Nemeth et al.1981), Bulgaria (Mihailova 1984), the United States (Hajdu et al. 1984; Herr et al. 1983; Yang et al. 1984), France (Lamarque and Perny 1985; Regnault 1985), Ukraine and Moldova (Bogdanova et al.1986), and Russia (Scripka et al. 1993).
In all these countries,
D. helianthi was assumed to be the sole causal agent of Phomopsis stem canker although several researchers suspected that the disease may be caused by more than one species of
Diaporthe (Aćimović and Štraser 1982; Herr et al. 1983; Yang and Gulya 1984).
In 2010,
an epidemic of Phomopsis stem canker occurred on sunflower in the U. S. states of
Minnesota, North Dakota and South Dakota, where over 75% of the sunflower production takes place in the United States. Using morphology and DNA sequence analyses, an undescribed species,
D. gulyae, was identified as causing Phomopsis stem canker in addition to
D. helianthi. A greenhouse experiment comparing the isolates of
D. helianthi and
D. gulyae
for their aggressiveness on sunflower showed that both are aggressive pathogens and the isolates varied significantly in their aggressiveness within
D. helianthi and
D. gulyae
(Mathew et al. 2015b). A year later, in Australia, where
D. helianthi is exotic, an outbreak of Phomopsis stem canker was reported affecting commercial sunflower fields and an investigation into the pathogens responsible for the outbreak revealed
D. gulyae and two previously undescribed species,
D. kongii and
D. kochmanii (syn.
D. sojae). Pathogenicity experiments in the greenhouse comparing the isolates of
D. gulyae,
D. kongii and
D. kochmanii
showed that
D. gulyae isolates were more aggressive on sunflower than the isolates of the other two pathogens (Thompson et al. 2011). In 2015, also from Australia, five new species were described pathogenic on sunflower (Thompson et al. 2015) and most recently,
D. novem was reported as a sunflower pathogen (Thompson et al. 2018). Also in 2015,
D. stewartii was confirmed causing Phomopsis stem canker in the U. S. state of Minnesota (Olson et al. 2017). Besides the above described species of
Diaporthe, ongoing research in Australia, Argentina and the United States has revealed at least fifteen species of
Diaporthe associated with sunflower that are either pathogenic or non-pathogenic (F. Mathew et al.,
unpublished;
S. Thompson et al.,
unpublished).
Symptoms and Signs
Infection of sunflower by
D. helianthi and possibly
D. gulyae is initiated during the vegetative growth stages of the sunflower plants. Symptoms include brown irregularly shaped spots on leaves which extend into large patches of dead tissue extending in from the leaf margin. The tissue surrounding the affected veins may be chlorotic, later becoming necrotic, and eventually the entire leaf dies prematurely (Figure 1). The infection spreads from the infected leaf down the petiole, which turns dark brown in color (Figure 2) and then on to the stem where a light-to-dark brown necrotic lesion develops at the node. As the infection develops, lesions increase in length over time (15 to 20 cm) and may girdle the stem (Figure 3) under favorable conditions. As infection continues to occur, the fungus degrades the pith tissue beneath the epidermis (Figure 4), thus causing the stem to become hollow and prone to lodging (Figure 5).
From leaf infection, the stem lesion takes approximately 25 to 30 days to form on the sunflower plant.
 |
 |
Figure 2 – Phomopsis infection progressing from leaf
down petiole.
|
Figure 3 – Characteristic
light brown stem lesions
centered at the nodes due
to early infection. Lesions will
grow and darken with age. |
 |
Figure 4 – Pith damage behind stem lesion caused by
D. gulyae |
 |
Figure 5 – Lodging of sunflower plants due to Phomopsis infection |
Symptoms of Phomopsis stem canker can be easily confused with those produced by other sunflower stem pathogens. For example, some species of
Diaporthe can produce a basal lesion on the sunflower plant similar to
Sclerotinia sclerotiorum (Sclerotinia mid-stem rot) and species of
Phoma (Phoma black stem). However, unlike Phomopsis stem canker, lesions of Phoma black stem are usually smaller, glossy, black colored, often shield shaped, (Figure 6), and generally do not cause pith degradation. Lesions of Sclerotinia mid-stem rot can look like that of Phomopsis stem canker, however, the Sclerotinia lesions are tan-colored and have black sclerotia forming inside the stem of the sunflower plant, which sometimes extruding out through the stem as the plant ages (Figure 7) (Gulya et al. 1997).
 |
 |
Figure 6 – Stem lesions
characteristic of Phoma
black stem |
Figure 7 – Stem lesions characteristic of Sclerotinia
mid-stem rot
|
Pathogen Biology
Diaporthehelianthi overwinters in infected crop debris as perithecia on the soil surface (Masirevic and Gulya 1992). As for
D. gulyae, perithecial stages of the fungus have rarely been observed under natural conditions (S. Thompson et al.,
unpublished).
Even so, the differentiation of the fungal ascogonia into perithecia is a slow process and occurs unevenly during spring months.
The perithecia are formed on cortical tissue; for
D. helianthi, they are
globular to spherical shaped,
yellow to black
colored, with a diameter of
400 µm and protruding long necks (350 to
600 µm). When the perithecia mature in spring, numerous asci are produced
which are globular to cylindrical shaped with sizes ranging from 47.5 to 57.5
µm
long x 7.5 to 12.0 µm wide
for
D. helianthi. The asci mature under an optimal temperature of 25°C (range from
15
to 30°C) and release ascospores to a height of approximately 3 mm above the surface of crop residue
under wet conditions and temperatures less than 10°C (Androsova et al. 2008).
At times, there may be a white mass or mucus drop containing ascospores produced on the apex of the rostrum of the perithecia (Maric et al. 1982; Yakutkin 1988).
Ascospores
of
D. helianthi are two-celled, colorless,
one-septate,
ellipsoidal, and range in size from
12.5 to 14.5
µm
long
by
3.2 to 4.5
µm wide (Muntañola-Cvetkovic
et al. 1989). The
D. helianthi ascospores are produced by perithecia for about 17 days (Li et al. 1985).
During disease development, pycnidia (170 to 3000 µm in diameter) may be produced by
D. helianthi and
D. gulyae in midsummer. Pycnidia are dark brown, globular, with occasional ostiolate beaks, and these are embedded in host tissue. When mature, pycnidia produce alpha conidia and/or beta conidia in amber colored droplets. Alpha conidia of
D. helianthi and
D. gulyae are hyaline, fusoid to ellipsoid in shape, and range in size from 5.5 to 10.0 µm long × 1.5 to 3.0 µm wide (Figure 8). While beta conidia of
D. helianthi are hyaline, thread-like, and range in size from 17.0 to 42.0 µm long by 0.5 to 2.0 µm wide (Figure 8), those of
D. gulyae have not been observed
in vitro (Mathew et al. 2015b;
Muntañola-Cvetkovic
et al. 1989; Thompson et al. 2015, 2011). The diameter of pycnidia and length/width ratio of conidia are variable among species and isolates of
Diaporthe causing disease on sunflower.
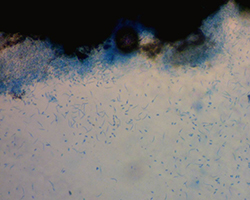 |
Figure 8 – Alpha (oval) and Beta (filiform)
asexual conidia. |
Disease Cycle and Epidemiology
The disease cycle begins with the production and release of ascospores from perithecia maturing on crop debris (Figure 9).
The ascospores are either blown by wind or splashed by rain onto the middle or lower leaves of sunflower plants throughout the growing season. These spores infect the margins of the older leaves of the sunflower plants via guttation droplets, where moisture accumulates. The initial lesions are developed on the sunflower leaves by the causal fungus when the relative moisture within canopy exceeds >90% for 36 h (Debaeke et al. 2017). However, on average, leaf lesions become visible 20 to 25 days after the ascospores cause infection for temperatures ranging from 20 to 25°C (Pinochet 1995). In addition to the temperature, rainfall from bud initiation (R1 growth stage; Berglund 2007) to flowering stage (R5
growth stage) of the sunflower plants is critical for successful leaf infection by the causal fungus.
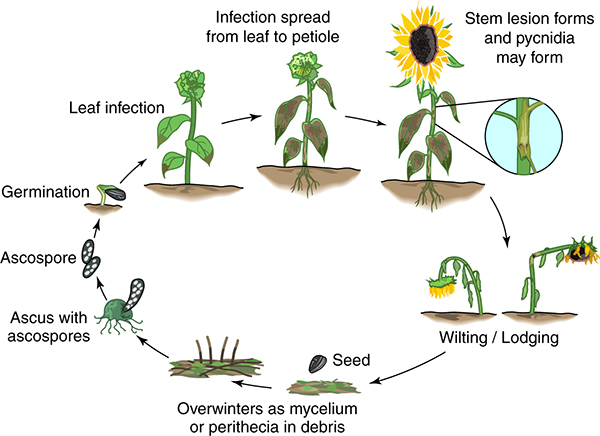 |
Figure 9 – Disease cycle of Phomopsis stem canker of sunflower |
After the leaves are infected, the mycelia
colonize the intercellular spaces, leaf veins and midrib to progress down the petiole and invade the stem systemically where an elongated tan-brown lesion is formed that is centered on axils
(Muntañola-Cvetkovic
et al. 1991).
The stem lesions are generally not observed on the sunflower plants until flowering (R5 growth stage).
As the stem is colonized, the hyphae progresses through the vascular system and spread via the collenchyma and cortex (Muntañola-Cvetkovic
et al. 1989). The cortex is covered with fungal hyphae, where pycnidial formation is initiated.
Yield losses occur when xylem and phloem tissues are invaded leaving the vascular system obstructed by the fungal hyphae. Subsequently, plants wilt and may also lodge mid-stem when unable to support the weight of the head (Figure 10) (Muntañola-Cvetkovic
et al. 1981).
 |
Figure 10 – Susceptible hybrid exhibiting severe Phomopsis
infection and lodging |
Phomopsis stem canker is believed to have only a primary disease cycle involving ascospores which travel considerable distances from inoculum reservoirs in crop debris, weed hosts or other infested sunflower fields. The ascospores infect sunflower plants in the field primarily due to lack of air movement and higher relative humidity from dense plant stands and canopy (Mihaljcevic et al. 1985).
Disease Management
Tillage: Species of
Diaporthe are known to survive in previous crop residue for up to five years (Masirevic and Gulya 1992). Burying the infected crop residue by tillage will drastically reduce the inoculum levels (Gulya et al. 1997), but the rate of breakdown of crop residue is dependent on winter weather conditions.
Rotation:
A rotational break of two to four years with non-hosts [e.g. small grains and corn (Zea mays L.)] can reduce
Diaporthe inoculum (Masirevic and Gulya 1992).
Plant density and nitrogen fertilization: Lower plant stands and lower levels of nitrogen can reduce the incidence and severity of Phomopsis stem canker (Debaeke and Moinard 2010). Debaeke and Moinard (2010) observed in a two-year field study that plots that received high N-fertilizer (120 kg N ha-1) had a significantly larger number of lesions on stems (girdling lesions) per plant when compared to plots with no N-fertilizer. In addition, plots with higher plant stand counts (8 plants/m2 = 80,000 plants ha-1) had higher number of lesions on stem of the sunflower plants when compared to plots with relatively lower plant density (5 plants/m2 = 50,000 plants ha-1) (Debaeke and Moinard 2010). In the United States, sunflower plant populations of 50,000 plants ha-1 or less are common.
Clean seed:
Although species of
Diaporthe
can be generally seed-borne, there is limited evidence of transmission of the sunflower pathogens via seeds (Fayret et al. 1996; Slyusar and Bochkarev 1997; S. Thompson,
unpublished).
Weed management:
In general, weeds are significant inoculum reservoirs of species of
Diaporthe associated with sunflowers in live (green) and dead (brown) bridges (Thompson et al. 2015). Among the weeds, wild sunflower (Helianthus annuus), Italian cocklebur (Xanthium italicum), common cocklebur (Xanthium strumarium), Noogoora burr (Xanthium pungens) and burdock (Arctium lappa) are hosts of
D. helianthi (Mihaljcevic et al. 1985;
Vrandecic et al. 2010).
Of D. gulyae, soybean (Glycine max L.), corn (Zea mays L.), Kochia (Kochia scoparia), lamb's quarters (Chenopodium album), Pennsylvania smartweed (Polygonum pensylvanicum), and saffron thistle (Carthamus lanatus) are hosts (Ash et al. 2010; Mathew et al. 2018b;
S. Markell and F. Mathew,
unpublished;
Thompson et al. 2015). Among the hosts of
D. gulyae, corn, Kochia and
lamb's quarters are symptomless hosts (S. Markell and F. Mathew,
unpublished; Thompson et al. 2015). Regardless, given that soybean, corn and weeds can serve as alternative hosts for
D. helianthi or D. gulyae and contribute to inoculum, it is important that these plant species be managed in a sunflower field.
Chemical control:
Fungicides have been used in Europe to manage Phomopsis stem canker. For example, in a study by
Debaeke et al. (2003), it was observed that application of a protectant fungicide at the bud initiation stage
(R1 growth stage)
reduced the incidence of Phomopsis stem canker. Additionally, Debaeke et al. (2003) recommended spraying of fungicides at a plant height of 50 to 70 cm using conventional/ground-driven sprayers. In the United States, although fungicides are labelled for management of sunflower foliar diseases, preliminary research by S. Markell and R. Harveson (unpublished) has indicated that fungicides alone are not sufficient for management of Phomopsis stem canker. Olson (2017) evaluated an integrated approach to manage Phomopsis stem canker that included combining fungicide application with sunflower hybrids having tolerance to the disease. Under severe disease pressure, Olson (2017) observed higher yields in plots with a single application ofpyraclostrobin [Headline; 6 fl oz A-1 = 0.43 liter
ha-1; FRAC Group 11 (Quinone outside Inhibitors (QoI)] at the bud initiation stage
(R1 growth stage)
on susceptible and tolerant hybrids when compared to non-treated control plots. After disease symptoms appear however, fungicide applications may not be effective (Olson 2017).
Genetic resistance:
Previous studies have suggested that resistance to
D. helianthi is quantitative in nature with additive effects (Viguié et al. 1999). In addition, it was proposed by Degener et al. (1999) that resistance factors in the leaf and stem are inherited independently. In the last decade, the sunflower germplasm fromex situ (USDA accessions from seed banks) and
in situ resources (wild species and land races) have been evaluated under field conditions for resistance to Phomopsis stem canker. For example, a total of 1,106 accessions of the USDA cultivated sunflower collection was evaluated by Gulya (1997) and 2% of the total accessions were observed to have resistance to the disease (defined as <10% of the plants infected). Besides the USDA cultivated sunflower collection, a number of wild
Helianthus species were reported to have resistance to
D. helianthi (Cuk 1982; Dozet 1990; Škorić 1984). Talukder et al. (2014) has identified 30 parental lines from Russia and Europe that have resistance to
D. helianthi. Recently, Mathew et al. (2018a) screened
54
USDA cultivated sunflower accessions for resistance to
D. helianthi
and
D. gulyae in the greenhouse.
Among the 54 accessions, 13 and four accessions were resistant to
D. helianthi and
D. gulyae, respectively, when compared to the susceptible USDA confection inbred ‘HA 288’ (Mathew et al. 2018a).
Significance
Phomopsis stem canker is a fungal disease responsible for high yield losses in sunflower in the United States and other countries of the world. Yield losses of 30 to 40% have been observed in commercial sunflower fields as a result of plant wilting and lodging from early
Diaporthe infection (Figure 10; Masirevic and Gulya 1992; Mathew et al. 2015b; Thompson et al. 2011). In a study by Pinochet and Estragnat (1996), it was observed that a girdling lesion on the stem can cause yield loss (~ 0.15 t ha-1 per 10% girdling lesion) on any genotype regardless of the level of resistance to Phomopsis stem canker. Besides yield, oil content may also be reduced by 15 to 25% due to Phomopsis stem canker (Aćimović 1986; Debaeke and Moinard 2010). Currently, options to manage Phomopsis stem canker are limited in the United States and other sunflower producing countries of the world. For example, in the United States, to manage the disease, only
FRAC Group 11 [(Quinone outside Inhibitors (QoI)]
fungicides are effective and partially resistant sunflower hybrids are available
(Olson 2017).
Further investigations are ongoing to determine optimal management strategies to manage Phomopsis stem canker of sunflower
in the United States.
Acknowledgments
This research project was funded, in parts, by the AFRI Foundational: Critical Agricultural Research and Extension (CARE) Program [Grant no. 2016-08651] from the USDA National Institute of Food and Agriculture. We thank Steve McKinley for the disease cycle.
Selected References
Aćimović, M. 1986. The effect of
Phomopsis sp. infection on grain yield and oil content of sunflower plants. Helia 9: 73–76.
Aćimović, M., and Štraser, N. 1982.
Phomopsis sp. -
novi parazit suncokreta (Phomopsis sp. - new parasite in sunflower).
Zaštita Bilja 33(2): 117-158 (English summary).
Androsova, V., Balakhnina, I., and Gulya, T. 2008. Structural aspects regarding formation and emission of
Diaporthe (Phomopsis)
helianthi ascospores. In: 17th International Sunflower Conference Program and Abstracts, Córdoba, Spain. June 8-12, 2008. pp. 103-107.
Ash, G. J., Sakuanrungsirikul, S., Anschaw, E., Stodart, B. J., Crump, N., Hailstones, D., and Harper, J. D. I. 2010. Genetic diversity and phylogeny of a
Phomopsis sp., a putative biocontrol agent for
Carthamus lanatus. Mycologia 102: 54– 61.
Berglund, D. R. (eds.) 2007. Sunflower production. North Dakota State University
Extension Service Report No. 25. Fargo, North Dakota, USA.
Bogdanova, V. N., Karadzova, L.V., and Steinberg, M.E. 1986.
Vovremia obnaruzit Phomopsis.
Seljskoe hozjaistvo Moldavii 12: 24-25.
Cuk, L. 1982. Using wild species in sunflower breeding. Uljarstvo 1: 23-27.
Debaeke, P., Bedoussac, L., Bonnet, C., Bret-Mestries, E., Seassau, C., Gavaland, A., Raffaillac, D., Tribouillois, H., Véricel, G., and Justes, E. 2017. Sunflower crop: environmental-friendly and agroecological. OCL 24(3): D304. DOI: 10.1051/ocl/2017020.
Debaeke, P., and Moinard, J. 2010. Effect of crop management on epidemics of Phomopsis stem canker (Diaporthe helianthi) for susceptible and tolerant sunflower cultivars. Field Crops Res. 115: 50-60.
Debaeke, P., Estragnat, A., and Reau, R. 2003. Influence of crop management on sunflower
stem canker (Diaporthe helianthi). Agronomie, EDP Sciences. 23 (7): 581-592.
Degener, J., Melchinger, E. A., and Hahn, V. 1999. Inheritance of resistance to
Phomopsis in sunflower: Study of leaf and stem resistance after artificial and natural infection. Helia 22(31): 105-115.
Dozet, B. M. 1990. Resistance to
Diaporthe/Phomopsis helianthi Munt. -Cvet. et al. wild sunflower species. In: Proceedings of the 12th Sunflower Research Workshop, Fargo, ND. January 9–10. 1990. National Sunflower Association, Bismarck, North Dakota, USA, pp. 86–88.
Fayret, J., Quenin, H., and Perny, A. 1996. Evolution of sanitary state of sunflower seeds. Consequences of capitulum attacks by
Phomopsis helianthi and
Phoma macdonaldii [French]. Phytoma -
La Défense des Végétaux 487: 37-40.
Gulya, T. J., Rashid, K. Y., and Masirevic, S. M. 1997. Sunflower diseases. In: Schneiter, AA (ed), Sunflower technology and production, American Society of Agronomy, Madison, Wisconsin, USA, pp. 313–319.
Gulya, T. J. 1997. Phomopsis stem canker resistance in USDA and commercial sunflower germplasm. Proc. 19th Sunflower Res. Workshop, Fargo, ND, USA, pp. 76-78.
Hajdu, F., Baumer, J. S., and Gulya, T. 1984. Occurrence of Phomopsis stem canker in Minnesota and North Dakota. Proc. Sunflower Research Workshop, Bismarck, North Dakota, p. 15.
Harveson, R. M., Markell, S. G., Block, C. C., and Gulya, T. J. 2016. Compendium of Sunflower Diseases, 1st ed. American Phytopathological Society, St. Paul, MN, USA.
Herr, L. J., Lipps, P. E., and Watters, B. L. 1983. Diaporthe stem canker of sunflower. Plant Dis. 67: 911-913.
Lamarque, C., and Perny, R. A. 1985.
Nouvelle maladie du tournesol:
Le phomopsis. Cultivar 179: 57-59.
Li, S., Maric, A., and Masirevic, S. 1985. Biological and epidemiological studies of
Phomopsis sp. (Diaporthe sp.) in sunflower. Zastita Bilja 36: 357-370.
Maric, A., Masirevic, S., and Li, S. 1982.
Prilog proucavanju Phomopsis spp. (Diaporthe spp.)
prowsrokovace sive pecevosti stabea suncokreta. Zastita Bilja 33(4): 403-419.
Masirevic, S., and Gulya, T. J. 1992.
Sclerotinia and
Phomopsis – two devastating sunflower pathogens. Field Crop Res. 30: 271– 300.
Mathew, F., Olson, T., Marek, L., Gulya, T., and Markell, S. 2018a. Identification of sunflower (Helianthus annuus) accessions resistant to
Diaporthe helianthi and
Diaporthe gulyae. Plant Health Prog. 19: 97–102.
Mathew, F., Gulya, T. J., Jordahl, J., and Markell, S. 2018b. First report of stem disease of soybean (Glycine max) caused by
Diaporthe gulyae in North Dakota. Plant Dis. 102: 240.
Mathew, F. M., Rashid, K. Y., Gulya, T. J., and Markell, S. G. 2015a. First report of Phomopsis stem canker of sunflower (Helianthus annuus) caused by
Diaporthe gulyae in Canada. Plant Dis. 99: 160.
Mathew, F. M., Alananbeh, K. M., Jordahl, J. G., Meyer, S. M., Castlebury, L. A., Gulya, T. J., and Markell, S. G. 2015b. Phomopsis stem canker: A reemerging threat to sunflower (Helianthus annuus) in the United States. Phytopathology 105: 990-997.
Mathew, F. M. 2014. Molecular characterization and pathogenicity of sunflower stem pathogens (Doctoral dissertation). Retrieved from ProQuest Dissertations Publishing Database. (Accession No. 3629478) (Last Accessed: 11/28/2017).
Mihaljcevic, M., Muntanola-Cvetkovic, M., Vukojevic, J., and Petrov, M. 1985. Source of infection of sunflower plants by
Diaporthe helianthi in Yugoslavia. Phytopathol. Z. 113: 334-342.
Mihailova, P. 1984.
Savremeni fitopatogeni problemi pri slanogleda. Rastitelna zascita 4(84): 11-14.
Miric, E. 2002. Pathological, morphological and molecular studies of a worldwide collection of the sunflower pathogens
Phomopsis helianthi and
Phoma macdonaldii. PhD thesis, University of Queensland, Australia.
Muntañola-Cvetkovic, M., Vukojevic, J., and Mihaljčević, M. 1991. The systemic nature of the sunflower disease caused by
Diaporthe helianthi. Can. J. Bot. 69: 1552-1556.
Muntañola-Cvetkovic, M., Vukojevic, J., and Mihaljčević, M. 1989. Pathohistology of
sunflower stems attacked by
Diaporthe helianthi. Can. J. Bot. 67: 1119-1125.
Muntañola-Cvetkovic´, M.,
Mihaljčević, M., and Petrov, M. 1985. On the identity of the causative agent of a serious
Phomopsis-Diaporthe disease in sunflower plants. Nova Hedwigia 34: 417–435.
Nemeth, F., Princzinger, G., and Vörös, J. 1981. New disease in Hungary. Magyar Mezogazdasag 48: 10-11.
Olson, T. R., Kontz, B., Gulya, T. J., Markell, S. G., and Mathew, F. M. 2017. First report of
Diaporthe stewartii causing Phomopsis stem canker of sunflower (Helianthus annuus) in Minnesota. Plant Dis. 101: 382.
Olson, T. R. 2017. Managing Phomopsis stem canker of sunflower using improved diagnosis and quantification of the causal pathogens. Theses and Dissertations. 1184.
http://openprairie.sdstate.edu/etd/1184
Pericin, D., Jarak, M., Anotv, M., and Dozet, B. 1994. Pectinase from
Phomopsis helianthi-the agent of sunflower stem canker. Helia 17(20): 21-30.
Pinochet, X., and Estragnat, A. 1996. Field evaluation of hybrid sensitivity to
Diaporthe helianthi: relationship between symptoms and yield losses. In: Proceedings of the 14th International Sunflower Conference, Beijing, China, pp. 777–780.
Pinochet, X. 1995.
Phomopsis et evaluation varie´tale de la sensibilite´. In: Le Page, R. (Ed.), Le Phomopsis du tournesol.
CETIOM, Paris, France, pp. 27–36.
Regnault, Y. 1985.
Premieres observations sur le Phomopsis du tournesol. Bullet Inf. Techn. CETIOM, Paris, France. 92: 13-20.
Santos, J. M., Correia, V. G., and Phillips, A. J. 2010. Primers for mating-type diagnosis in
Diaporthe and
Phomopsis: their use in teleomorph induction in vitro and biological species definition. Fungal Biol. 114: 255–270.
Scripka, O. V., Sheluhin, V. I., Petina, V. V., and Serebryakova, P. P. 1993.
Phomopsis in sunflower. Crop Prot. 8: 24-25.
Škorić, D. 1984. Genetic resources in the
Helianthus genus. In: D. Škorić, editor, Proceedings of the International Symposium on Science and Biotechnology for an Integral Sunflower Utilization, Bari, Italy. October 25, 1984. pp. 37–73.
Slyusar, E. L., and Bochkarev, N. I. 1997.
Phomopsis and seed quality.
Zashchita Karantin Rastenić 8: 30–31.
Su, L., Maric, A., and Masirevic, S. 1985. Biological and epidemiological studies of
Phomopsis sp. (Diaporthe sp.) in sunflower. Zaštita Bilja 36: 357-370.
Talukder, Z. I., Hulke, B. S., Marek, L. F., and Gulya, T. J. 2014. Sources of resistance to sunflower diseases in a global collection of domesticated USDA Plant Introductions. Crop Sci. 54: 694-705.
Thompson, S. M., Tan, Y. P., Neate, S. M., Grams, R. M., Shivas, R. G., Lindbeck, K., and Aitken, E. A. B. 2018.
Diaporthe novem isolated from sunflower (Helianthus annuus) and other crop and weed hosts in Australia. Eur. J. Plant Pathol. 91: 1-9.
Thompson, S.M., Tan, Y.P., Shivas, R.G., Neate, S.M., Morin, L, Bissett, A., and Aitken, E.A.B. 2015. Green and brown bridges between weeds and crops reveal novel
Diaporthe species in Australia. Persoonia 35: 39-49.
Thompson, S. M., Tan, Y. P., Young, A. J., Neate, S. M., Aitken, E. A. B., and Shivas, R. G. 2011. Stem cankers on sunflower (Helianthus annuus) in Australia reveal a complex of pathogenic
Diaporthe (Phomopsis) species. Persoonia 27: 80–89.
Viguié, A., Vear, F., and de Labrouhe, D. T. 1999. Interactions between French isolates of
Phomopsis/Diaporthe helianthi Munt. -Cvet. et al. and sunflower (Helianthus annuus L.) genotypes. Eur. J. Plant Pathol. 105: 693-702.
Vrandecic, K., Jurkovic, D., Riccioni, L., Cosic, J., and Duvnjak, T. 2010.
Xanthium italicum,
Xanthium strumarium, and
Arctium lappa as new hosts for
Diaporthe helianthi. Mycopathologia 170: 51–60.
Yakutkin, V. I. 1988. Methods for identification and evaluation of sunflower
Phomopsis. In: Collection of methodological recommendations in crop protection. St. Petersburg, Russia, pp. 191-207.
Yang, S. M., and Gulya, T. J. 1984. Groups of
Diaporthe/Phomopsis isolates obtained from cultivated sunflower. Phytopathology 74: 868.
Yang, S. M., Berry, R. W., Luttrell, L. E. S., and Vongkaysone, T. 1984. A new sunflower disease in Texas caused by
Diaporthe helianthi. Plant Dis. 68: 254-255.
