Soybean Rust
Phakopsora pachyrhizi and
P. meibomiae
Soybean and kudzu are the most important hosts, but there are over 90 other hosts known for P. pachyrhizi. Some of the hosts are listed in Table 1.

Soybeans infected and not infected with Asian soybean rust, caused by
Phakopsora pachyrhizi, in a fungicide trial in Attapulgus, GA, 2006. (Photo by R. C. Kemerait, Jr.) |
Soybean rust caused by
P. pachyrhizi has been a serious disease in Asia for many decades. It appeared in Africa in 1997, and in the Americas in 2001. Before it was first found in the continental USA in late 2004, probably brought in by a hurricane, it was considered such a threat that it was listed as a possible weapon of bioterrorism. Soybean rust cannot overwinter in areas with freezing temperatures, but it can spread by wind rapidly over such large distances, its development can be so explosive, and it can cause such rapid loss of leaves that it is now one of the most feared diseases in the world's soybean-growing areas.
Symptoms and Signs
The first symptoms of soybean rust caused by
Phakopsora pachyrhizi begin as very small brown or brick-red spots on leaves (Figure 2). Symptoms caused by
P. meibomiae are similar to those of
P. pachyrhizi, but this lesson will focus on
P. pachyrhizi because most of the research and observations have been made with this species. In the field, these spots usually begin in the lower canopy at or after flowering, although seedlings can be infected under certain circumstances. Often the first lesions appear toward the base of the leaflet near the petiole and leaf veins. This part of the leaflet probably retains dew longer, making conditions more favorable for infection. Lesions remain small (2-5 mm in diameter), but increase in number as the disease progresses. Pustules (Figure 3), called uredinia, form in these lesions, mostly on the lower leaf surface, and they can produce many urediniospores. The raised pustules can be seen with the unaided eye, especially when sporulating (Figure 4). Even though the lesions are small, each lesion often has several pustules (uredinia) (Figure 5). Lesions can be completely covered in urediniospores when the pustules are active (Figure 6). Soybean rust urediniospores are pale yellow-brown to colorless, with an echinulate (short spines) surface ornamentation (Figures 7 and 8). This coloration is different from many other rust pathogens whose spores are often reddish-brown (rust colored). Germination of
P. pachyrhizi urediniospores occurs through an equatorial (central) pore, producing a germ tube that ends in an appressorium, which the fungus uses to penetrate the host directly or through a stoma (Figure 9).

Figure 1 |

Figure 2 |

Figure 3 |

Figure 4 |

Figure 5 |

Figure 6 |
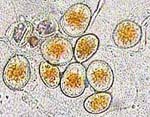
Figure 7 |
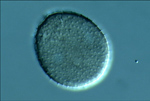
Figure 8 |
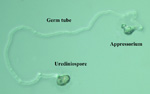
Figure 9 |
As more and more lesions form on a leaflet, the affected area begins to yellow, and eventually the leaflet falls from the plant (Figure 1). While soybean rust usually begins in the lower canopy, it quickly progresses up the plant until all of the leaves have some level of disease. Severely diseased plants may become completely defoliated. The loss of effective leaf tissue results in yield reductions from both fewer and smaller seed. Yield losses as high as 30 to 80% have been reported, but the amount of loss depends on when the disease begins and how rapidly it progresses. Besides leaves, soybean rust can also appear on petioles, stems, and even cotyledons, but most rust lesions occur on leaves.
Lesions may be either tan (Figure 10) or red-brown (Figure 11). Tan lesions have many pustules that produce numerous urediniospores. Red-brown lesions, thought to be a moderate resistance reaction, have only a few pustules that produce only a few urediniospores. As will be discussed in the Disease Management section, this lesion type depends on the strain of the pathogen, and may appear on the same leaf with tan lesions, or tan lesions may turn red-brown with age. Symptoms and signs on other hosts, such as kudzu, are similar, although lesion size may differ.
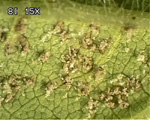
Figure 10 |

Figure 11 |
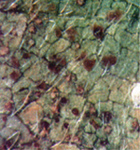
Figure 12 |

Figure 13 |
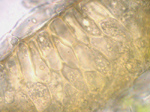
Figure 14 |
| |
As pustules age, they may turn black (Figure 12). This is caused by the formation of a layer of teliospores in the pustules, turning pustules from uredinia into telia (Figure 13 and 14). Teliospores have two functions: survival of the fungus in the absence of a living host (overseasoning) and sexual reproduction. The thick walls of the teliospores protect the fungus from the environment and attack by other organisms. In rusts, the teliospores germinate forming a basidium and four basidiospores during which sexual recombination occurs. Germination of
P. pachyrhizi teliospores has been observed only in the laboratory and does not seem to make a significant contribution to the perpetuation of this disease in the field.
Pathogen Biology
There are two closely related fungi that cause rust on soybean:
Phakopsora pachyrhizi, sometimes referred to as the Asian or Australasian soybean rust pathogen, but which now also occurs in the western hemisphere, and
P. meibomiae, the so-called New World soybean rust pathogen, which is found only in the western hemisphere. Except for a few minor characteristics, the two fungi appear morphologically identical, but
P. pachyrhizi is much more aggressive on soybean than
P. meibomiae. To date,
P. meibomiae has not been documented to cause significant yield losses in Central and South America. The two species can be distinguished by using DNA analysis protocols. Like other rusts, the soybean rust pathogens are obligate parasites that require a living host to grow and reproduce. They can survive away from the host as urediniospores for only a few days under natural conditions.
Both soybean rust pathogens, to the best of our knowledge, produce only two types of spores: urediniospores and teliospores (Figure 15). This contrasts with other rusts, which can have up to five spores stages (for example, wheat stem rust). For soybean rust, like most rusts, the uredinial stage is the repeating stage. This means that urediniospores can infect the same host on which they were produced (soybean) during the same season. Epidemics can develop quickly from only a few pustules because spore-producing pustules are produced in as little as 7 to 10 days after infection, and each pustule can produce hundreds of urediniospores.
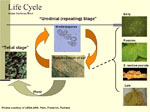
Figure 15 |
Teliospores are produced in old lesions, but they do not appear to germinate in nature, and no alternate host, nor aecia or spermogonia are known. Without germination of teliospores, sexual reproduction cannot take place. Lack of sexual reproduction should limit variability of the rust fungus, but nevertheless there is substantial variability in
P. pachyrhizi with respect to virulence. This has limited the use of single genes for resistance in soybean, because in a short time new isolates of the pathogen arise that overcome the resistance gene. It is not known how this variability originates in
P. pachyrhizi. Wheat stripe rust,
Puccinia striiformis, has a similar life cycle as
P. pachyrhizi with no functioning telial stage and therefore no sexual reproduction, but has many races. It may be that each resistance gene is so specific that a single mutation in the right gene of the fungus allows it to be virulent on hosts with the new resistance gene.
Epidemiology
Soybean rust epidemics begin with the arrival of airborne inoculum (urediniospores). This pathogen is unique among rusts because it has many alternative hosts (Table 1), which may serve as sources of inoculum.
Alternative hosts are other plants that can become infected with the same pathogen, but are not required to complete the pathogen's life cycle. Alternative hosts are not to be confused with
alternate host, which is a plant other than the principal host, that is needed for a pathogen to complete its life cycle. In frost-free areas, such as South America, Central America, the Caribbean basin, southern Texas, and Florida, the inoculum source could be nearby on volunteer soybean plants, kudzu, or some other alternative host. In areas that experience frost, such as the Midwestern United States, inoculum must be blown in from over-wintering sources that may be hundreds of miles away. Re-introduction of obligate pathogens into a distant region occurs with several other diseases, such as wheat stem rust and downy mildews, e.g. blue mold on tobacco. Because spores of
P. pachyrhizi are sensitive to ultraviolet radiation, long distance movement of these rust spores probably occurs in storm systems where clouds protect the spores from the sun.
Table 1. Known hosts of Asian soybean rust caused by
Phakopsora pachyrhizi. Information courtesy of Kent Smith, USDA/ARS.
|
COMMON NAME |
SCIENTFIC NAME |
| Bean, Common, Dry (field, kidney, navy, pinto) * |
Phaseolus vulgaris var.
vulgaris |
| Bean, Common, Succulent (garden, green, snap, and wax) * |
Phaseolus vulgaris var.
vulgaris |
| Bean, Fava or Broadbean |
Vicia faba |
| Bean, Lablab or Hyacinth * |
Lablab purpureus |
| Bean, Lima * |
Phaseolus lunatus var.
lunatus |
| Bean, Mung * |
Vigna radiata |
| Bean, Scarlet Runner * |
Phaseolus coccineus |
| Bean, Winged or Goa |
Psophocarpus tetragonolobus |
| Bean, Yam * |
Pachyrhizus ahipa, P. erosus |
| Blackeyed Pea, Cowpea or Yardlong Bean * |
Vigna unguiculata |
| Calopo |
Calopogonium mucunoides |
| Clover; Alyce or Oneleaf |
Alysicarpus vaginalis |
| Clover, Crimson |
Trifolium incarnatum |
| Clover, Hop |
Trifolium aureum |
| Clover, Lappa |
Trifolium lappaceum |
| Clover, White |
Trifolium repens |
| Crotalaria * |
Crotalaria anagyroides, C. spectabilis |
| Crownvetch |
Securigera varia |
| Fenugreek |
Trigonella foenum-graicum |
| Florida Beggarweed* |
Desmodium tortuosum |
| Kudzu * |
Pueraria montana var.
lobata |
| Lespedeza |
Lespedeza spp.,
Kummerowia striata, K. stipulaceae |
| Lupines |
Lupinus spp. |
| Medic |
Medicago spp. |
| Milk Vetch |
Astragalus cicer, A. glycyphyllos |
| Pea, garden and field |
Pisum sativum |
| Peatree or Colorado River Hemp (Sesbania) |
Sesbania exaltata |
| Pigeon Pea * |
Cajanus cajan |
| Siratro * |
Macroptilium atropurpureum |
| Soybean (including edamame) * |
Glycine max |
| Swordbean * |
Canavalia gladiata |
| Trefoil |
Lotus spp. |
| Urd or Black Gram * |
Vigna mungo |
| Wild Soybean * |
Neonotonia wightii |
| Woolypod Vetch |
Vicia villosa subsp.
varia |
| Yellow Sweet Clover |
Melilotus officinalis |
| * Includes field observations of infection, in addition to infection resulting from artificial inoculation |
Once viable spores have landed on the leaf surface of a suitable host, infection and subsequent epidemic development are dependent on environmental conditions. Generally, infection occurs when leaves are wet and temperatures are between 8°C and 28°C, with an optimum of 16°C to 28°C. At 25°C, some infection occurs in as little as 6 hours of leaf wetness, but 12 hours are optimal. After infection, lesions and pustules with urediniospores can appear within 7 or 8 days, and the next infection cycle is set to begin. This short life cycle means that, under the right conditions, soybean rust epidemics can quickly build up from almost undetectable levels to very high levels. Soybean rust epidemics can progress from below detectable levels to defoliation within a month. Epidemics may seem to progress even faster than that, because early infections occur in the lower canopy and are hard to find. Besides the environment, plant age affects soybean rust epidemics. Usually, rust lesions are not found on soybean until flowering, unless there are high inoculum levels early in the season. This may be due to greater susceptibility of plants to rust as the host enters the reproductive stages, it may be because in lower parts of the canopy spores are more protected from UV radiation, or it may be because conditions in the canopy become more humid as the canopy closes. In any event, lesions can form at any growth stage, but major increases in disease do not occur until after flowering.
Disease Management
There are three basic management tactics that can play a role in reducing soybean rust epidemics: fungicides, genetic resistance, and cultural practices. At present, fungicides are the only highly effective tactic (Figure 16), but long-term management will probably depend more on resistance, in combination with fungicides and changes in cultural practices.
At present, the most effective means of managing soybean rust is the use of fungicides (Figure 1). However, to be effective, selecting the right fungicide and applying it at the right time are crucial. Several fungicides are registered in the US for soybean rust control, and most can be classified into three groups: chloronitriles, strobilurins, and triazoles (Figure 17). Chlorothalonil is the one chloronitrile fungicide registered for soybean rust control. Its protectant mode of action affects many biochemical pathways in the pathogen, but it is not taken up by the plant, not even by the cuticle. As a result, it is more subject to weathering than the strobilurins or the triazoles and complete coverage of the leaf surface is critical. To be effective, chlorothalonil may need to be reapplied several times if new growth or weathering occurs.

Figure 16 |

Figure 1 |
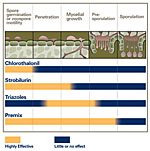
Figure 17 |
Strobilurin fungicides are modeled after a natural antifungal compound, strobilurin, produced by certain mushrooms. Strobilurins (also known as QoI fungicides) inhibit mitochondrial respiration in the pathogen. Strobilurins are typically absorbed by the cuticle, and act as protectant fungicides (http://admin.apsnet.org/edcenter/advanced/topics/Pages/StrobilurinFungicides.aspx). A protectant fungicide prevents infections from taking place, but it has little effect on disease development once infection has occurred (Figure 17). Therefore, to be effective, protectants like the strobilurins must be applied before infection occurs. Depending on the rate applied, strobilurins are effective for up to 2 weeks after an application, but they will not protect newly developing leaves. Strobilurins control a broad range of soybean pathogens.
Triazoles inhibit sterol production, which disrupts cell membrane function in the pathogen. Triazoles are absorbed and translocated upward in the plant. While they generally do not prevent infection, the triazoles can kill the fungus in the plant and prevent pustules and spores from forming (Figure 17). The extent to which these chemicals are translocated depends on the triazole, but all of them move up the plant into new growth to one degree or another. Still, systemicity of triazoles in plants is incomplete and does not approach the level of systemicity associated with certain herbicides or insecticides. Triazoles are effective for 3 or 4 weeks after application and give some protection to new growth. While highly effective against rust, the triazoles are not as effective as the strobilurins against other soybean pathogens. Some fungicide products (premixes) contain both a triazole and a strobilurin. The premixes provide protection against a broader range of pathogens and reduce the possibility of pathogens developing resistance to either product.
The number of applications required for disease control depends on the compounds used, when the rust epidemic starts, and the favorability of the weather conditions. Even with triazoles, which are effective for the longest period of time, two applications are often needed to control soybean rust. In some locations in Brazil, high levels of inoculum early in the season result in rust epidemics starting well before flowering, thus forcing growers to make as many as five fungicide applications in order to control the disease. Such early disease onset and the early need for fungicide application are unlikely in most of the US. However, rust could start as early as flowering (R1) and require an additional spray before harvest. It is generally felt that, once the plants reach the R6 growth stage (when seeds have filled the pod), most of the yield has been achieved and controlling rust beyond that point is not economical. One concern with multiple applications of the same fungicide is the development of fungicide resistant pathogen strains. While fungicide resistance in
P. pachyrhizi has not been reported, other fungal pathogens may be affected and growers should try not to spray the same fungicide consecutively. Fungicide labels may restrict the number of times a particular compound or class of compounds can be applied within a season to reduce the possibility of resistance developing.
The key to effective control of soybean rust with fungicides is application timing. This is especially important in areas of the US where the soybean rust pathogen must be reintroduced each year. The introduction or reintroduction will probably occur at different times in different years or not at all in some years. All of the fungicides, even the systemic triazoles, are most effective when applied just before the rust epidemic starts in the field. From tests in South America, if disease incidence reaches 10% in the lower canopy before the first application, fungicides will not completely control soybean rust, and some yield loss will result if weather conditions are favorable. Such low levels of disease are difficult to detect so growers need an early warning system that predicts the onset of disease early enough so that they have time to apply fungicides to all of their fields. Application decisions, equipment, and technique can all greatly impact the level of rust control achieved.
At present, the most reliable early detection method is the use of "sentinel plots." These are small plots (primarily soybean, but kudzu or another susceptible host also may be used) planted several weeks before the commercial crop, and often use early maturing cultivars. Both the early planting and the early maturity of the cultivars results in the sentinel plots flowering 1 to 3 weeks before the commercial crop. Since soybean rust usually develops after flowering, the disease can be observed in these sentinel plots a week or two before being found in adjacent commercial fields. This early warning gives growers in the area time to apply a protective fungicide treatment. Sentinel plots have been established throughout the soybean and dry bean production areas of the US. Information from these plots is uploaded weekly into a USDA website (www.sbrusa.net) where maps are generated showing rust activity in the country (Figure 18). This site also includes state and national commentaries, disease forecasts, and other pertinent information.
Figure 18 |
Besides the sentinel plot findings, extension specialists in each state also include state-specific commentary on soybean rust and the need for control measures. In addition, rust information from all of the state plant diagnostic clinics is networked together, and new finds of soybean rust are included on the site. Information from this USDA website can be used by growers and scientists to see where rust is active and to determine if their area is threatened by the disease. In addition to the USDA website, information is also available on many state Cooperative Extension Service's websites and on several agricultural industry websites. Information about soybean rust in Argentina can be found at
http://www.sinavimo.gov.ar/ and in Brazil at
http://www.cnpso.embrapa.br/alerta/. A partial list of websites can be found in the Selected References section of this lesson.
Several experimental early warning and disease forecasting systems are under development. These models relate a variety of weather, crop and disease conditions to spore movement, spore deposition, and infection. Some of the factors included in these models are sources of inoculum, wind direction and speed, temperature, humidity, leaf wetness, sunlight intensity, and crop developmental stage. These models are currently being used to indicate where and when scouting efforts should be intensified.
Another method of early detection of soybean rust is spore trapping, in which two strategies are being assessed. One traps windblown spores on glass slides coated with petroleum jelly (Figure 19). The spores are examined microscopically, and the presence and number of soybean rust-like spores noted. At this time, microscopic examination can only identify spores that resemble the soybean rust pathogen because it is not currently possible to identify
P. pachyrhizi with certainty by simply examining the urediniospores. More conclusive identification of urediniospores of
P. pachyrhizi is being developed by using labeled antibodies and polymerase chain reaction (PCR) protocols.

Figure 19 |

Figure 20 |
The other spore trapping approach involves collecting and filtering rainwater and then uses PCR to determine the presence of
P. pachyrhizi on the filters (Figure 20). It is thought that long-distance spread of urediniospores occurs when storms pick up the spores and then deposit them in rainwater at distant locations. Because this technique uses species-specific molecular markers, positive findings are thought to be more reliable. In 2005 and 2006, both air and rain sampling found
P. pachyrhizi or
P. pachyrhizi-like spores over a wide area, far from where soybean rust was active. While neither approach can determine if the spores arrived alive, they do indicate that this pathogen has the potential to spread widely and quickly.
Genetic Resistance
Soybean plants respond to infection by
P. pachyrhizi by producing either tan, red-brown, or no lesions at all. Tan lesions produce many pustules with many spores (Figure 10). Red-brown lesions produce a few pustules with limited spore production, and no pustules or spores are produced where no lesions are formed (Figure 11). It is thought that these responses represent susceptible, moderate, or highly resistant reactions, respectively. High levels of resistance are usually associated with one or a few dominant genes. There are four known dominant genes for resistance to soybean rust,
Rpp1 through
Rpp4. While these dominant genes confer high levels of resistance and are relatively easy to incorporate into new soybean cultivars, they are not effective against all races of
P. pachyrhizi. Deployment of varieties with new resistance genes is usually followed in a few years by the emergence of races of
P. pachyrhizi that are virulent on them. This high degree of variability in the soybean rust pathogen is common in many rusts [see wheat stem rust] and requires the frequent discovery and incorporation of new sources of resistance. Currently, isolates of
P. pachyrhizi exist that are virulent on each of the four known genes for resistance.

Figure 10 |

Figure 11 |
Another approach is the use of moderate resistance. Moderate resistance is usually conferred by a number of genes, each contributing a little to the overall resistance of the cultivar. This type of resistance often is effective against all races of a pathogen, but it is more difficult to incorporate into cultivars and does allow some disease and yield loss. Moderately resistant cultivars have been developed in Asia, but adapted varieties with this type of resistance are not yet available in the US or South America. Ultimately, moderate resistance may be used in combination with cultural practices and fungicides when needed.
Cultural practices
There are several cultural practices that may help manage soybean rust. In most areas of the US where rust must be introduced each year for an epidemic to occur, changing planting and harvest dates may avoid disease. Planting early with an early maturing cultivar may avoid the rust until the crop has either been harvested or is so far along that the disease will have little impact on yield. Planting dates may also be delayed so that the vulnerable reproductive period occurs during dry conditions that do not favor rust. In areas where the weather is marginal for rust development, wider row spacing along with lower plant populations may hasten canopy drying, thus reducing the dew period enough to prevent or at least slow disease development. It may also allow better fungicide penetration into the canopy, increasing the effectiveness of chemical control. Research is needed to confirm this. However, because the more open canopy provides less weed suppression, weed problems may be more severe with this strategy, and this method is unlikely to affect rust significantly if weather conditions are very favorable for the disease. Adjusting soil fertility, particularly potassium and phosphorus levels, may help increase disease resistance, but there is little research in this area yet. While it is unlikely that cultural control measures alone will be enough to control soybean rust, they may increase the effectiveness of host resistance or fungicide applications.
Significance
Soybean rust is one of the most important soybean diseases worldwide. Soybean, a major crop both in the US and the world, is high in vegetable oil and protein (approximately 20 and 40%, respectively) and provides 57% of the vegetable oil consumed worldwide and 68% of the vegetable protein. According to the American Soybean Association, the US produced 38% of the world soybean crop in 2006, worth over $19 billion, with Brazil and Argentina producing 24 and 19% of the world crop, respectively. Because of the lack of plant resistance, the explosive nature of the disease, and the high potential yield losses (30 to 80%), soybean rust has long been viewed as a serious threat to soybean production in both North and South America. The threat of soybean rust was so serious that
Phakopsora pachyrhizi was included on a list of 'select agents' in the 2002 USA Bioterrorism Act, along with other biological agents such as those that cause anthrax and hemorrhagic fever. Select agents are pathogens of humans, animals, or plants that have the potential to be used as weapons of terrorism.
Soybean rust caused by
P. pachyrhizi was first reported in Japan in 1902 and was limited to Asia and Australia until 1997 when it was found in Uganda. From Uganda it spread to Zimbabwe (1998) and then to South Africa (2001). In 2001, soybean rust was found in Paraguay. Since most of the world's soybean production occurs in North and South America, the introduction of rust into Paraguay posed a significant threat. Soybean rust was reported in Brazil and northern Argentina in 2002. By 2003, soybean rust was occurring in most soybean producing areas in Brazil as well as Bolivia. In the summer of 2004, rust was reported in Columbia, and in November of that year it was found for the first time in the continental US in Louisiana and then, within a short time, in 8 other southern states (Figure 21). Soybean rust probably entered the continental US from Columbia with hurricane Ivan, which made landfall in September 2004.
Figure 22 illustrates the estimated spore load carried by hurricane Ivan and the geographic distribution of spore deposits. Since then,
P. pachyrhizi appears to have established itself permanently on kudzu in Florida, and the area where it is found during the growing season has increased. In 2005, soybean rust was active primarily in the southeastern US (Figure 23), but some late finds were found on kudzu as far north as Kentucky and North Carolina. Soybean rust was found in Mexico in the early spring of 2006 and in an isolated field near Brownsville, TX.. Throughout the first half of the 2006 growing season, soybean rust was confined to the southeastern US, but it was not very active even there because of the unusually hot, dry weather. However, as rainfall increased, so did soybean rust, especially in Louisiana and along the southeast coast of the US. By the end of the season, soybean rust was found along the Mississippi River into southern Illinois and Indiana and as far north as West Lafayette, IN (Figure 24). In 2007, drought in the southeastern US limited soybean rust development throughout most of the season, but high rainfall in Louisiana, Texas, Oklahoma, and Kansas favored rust in these areas (Figure 25). Rust appeared for the first time as far north as Iowa in 2007. Although these late-season infections did not cause yield loss, it did show that under the right conditions, soybean rust can spread quickly and is a threat to the major soybean growing areas of the US.
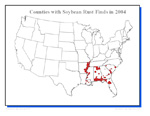
Figure 21 |
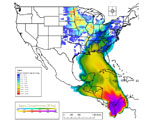
Figure 22 |
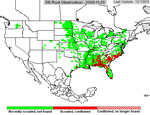
Figure 23 |
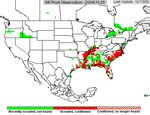
Figure 24 |
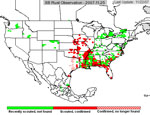
Figure 25 | |
Note: The first introduction of Asian soybean rust caused by
P. pachyrhizi in the US occurred in Hawaii in 1994, but this did not impact the major soybean growing areas in the continental US. Also, soybean rust was found in Puerto Rico in the mid-1970's, but later analysis showed that this rust was caused by
P. meibomiae and not
P. pachyrhizi.
The introduction of Asian soybean rust into the continental US sparked a nationwide effort by research and extension scientists to prepare for rust epidemics. These efforts include a network of sentinel plots that track the occurrence of soybean rust nationally on a weekly basis, a coordinated effort to label fungicides for soybean rust, training of "first detectors" on how to recognize soybean rust, and other extension and research activities. As a result, this disease has sparked immense media interest. Some news headlines follow.
"Soybean Checkoff Builds Defense Against Rust"
Soy News:
http://www.arspb.org/publications/soynewspdfs/SoyNews09-04.pdf
"RUST BELT CINCHES UP"
AgWeb.Com:
http://www.agweb.com/get_article.aspx?src=&pageid=108090
"Sen. Feingold urges action against soybean rust"
CropChoice.com:
http://www.cropchoice.com/leadstry9205.html?recid=2337
The Asian soybean rust work that has been conducted in the United States to date would not have been possible without a concerted and cooperative effort from Land Grant Universities, the U.S. Department of Agriculture, State Soybean Check-Off Boards, and Industry.
Selected References
APS Press Release. 2004. Plant Pathologists Offer Soybean Rust Identification and Management Tips.
http://apsnet.org/publications/apsnetfeatures/Pages/SoybeanRustIdentification.aspx.
Bromfield, K.R. 1984. Soybean Rust. Monograph 11. The American Phytopathological Society, St. Paul, MN.
Dorrance, A.E., M.A. Draper, and D.E. Hershman, Editors. 2007 (revised). Using Foliar Fungicides to Manage Soybean Rust:
http://www.oardc.ohio-state.edu/SoyRust/index.htm
Dunphy, J., D. Holshouser, D. Howle, P. Jost, B. Kemerait, S. Koenning, J. Mueller, P. Phipps, S. Rideout, L. Sconyers, E. Stromberg, P. Wiatrak, and A. Wood. 2006. Managing Soybean Rust in the Mid-Atlantic Region:
http://cipm.ncsu.edu/ent/SSDW/RUSTX3.pdf
Hernández, J.R. 2004. Systematic Botany & Mycology Laboratory, ARS, USDA. Invasive Fungi. Asian soybean rust. Retrieved October 2, 2007, from
http://nt.ars-grin.gov/sbmlweb/fungi/index.cfm
Miles, M.R., R.D. Frederick, and G.L. Hartman. 2003. Soybean Rust: Is the U.S. Soybean Crop At Risk?
https://www.apsnet.org/edcenter/apsnetfeatures/Pages/SoybeanRust.aspx.
Sinclair, J.B., and G.L. Hartman. 1999. Soybean Rust. Pages 25-26. In Compendium of Soybean Diseases, Fourth Edition. Eds. G.L. Hartman, J.B. Sinclair, and J.C. Rupe, APS Press, St. Paul, MN.
Sconyers, L.A., R.C. Kemerait, J. Brock, D.V. Phillips, P.H. Jost, E.J. Sikora, E. Gutierrez-Estrada, J.D. Mueller, J.J. Marois, D.L. Wright, and C.L. Harmon. 2006. Asian Soybean Rust Development in 2005: A Perspective from the Southeastern United States.
http://apsnet.org/edcenter/apsnetfeatures/Pages/SoybeanRustDev.aspx.
Websites
National-Public
Aerobiology at Penn State:
http://www.ceal.psu.edu/
APHIS Site:
http://www.aphis.usda.gov/plant_health/plant_pest_info/soybean_rust/index.shtml
APS site:
http://www.plantmanagementnetwork.org/infocenter/topic/soybeanrust/
USDA PIPE:
http://www.sbrusa.net/
International-Public
Argentine website:
http://www.sinavimo.gov.ar/
Brazilian website:
http://www.cnpso.embrapa.br/alerta/
State and Regional-Public
Extension Disaster Education Network:
http://www.eden.lsu.edu/Issues_View.aspx?IssueID=E614E265-F3EE-4777-BC3B-9B20DE9C2120
North Central Plant Diagnostic Network:
http://www.ncpdn.org/
Plant Health Initiative:
http://www.plantmanagementnetwork.org/pub/search/topsearch.asp?page=http://planthealth.info/rust_basics.htm
Southern Extension:
http://www.ces.ncsu.edu/depts/pp/notes/Soybean/soy008/soy008.htm
Industry
BASF:
http://www.soybeanrustinfo.com/
StopSoybeanRust:
http://www.stopsoybeanrust.com/mc_home.asp
Syngenta Crop Protection.
http://www.farmassist.com/soybeanrust/
