P. D. Esker1, A. H. Sparks1, G. Antony1, M. Bates2, W. Dall' Acqua1, E. E. Frank1, L. Huebel3, V. Segovia1, and K. A. Garrett1
1Dept. of Plant Pathology, Kansas State University, Manhattan, KS
2Dept. of Horticulture, Forestry, and Recreation Resources, Kansas State University, Manhattan, KS
3Dept. of Mathematics, Kansas State University, Manhattan, KS
Current address of P. D. Esker: Dept. of Plant Pathology, University of Wisconsin, Madison, WI, USA
Esker, P.D., A.H. Sparks, G. Antony, M. Bates, W. Dall' Acqua, E.E. Frank, L. Huebel, V. Segovia, and K.A. Garrett, 2007. Ecology and Epidemiology in R: Modeling dispersal gradients.
The Plant Health Instructor. DOI:10.1094/PHI-A-2008-0129-03.
Student Learning Goals
After completion of this module:
- Students will understand how R can be used to model dispersal and disease gradients.
- Students will be able to:
- use R to compare different dispersal gradient models,
- use R to compare and analyze primary versus secondary gradients,
- run simulations in R that illustrate how an epidemic changes in space and time.
Feedback
We would appreciate feedback for improving this paper and information about how it has been used for study and teaching. Please send your feedback to
kgarrett@ksu.edu. Please include the following text in the e-mail subject line, "Feedback on R Modules", to make sure your comments are received.
The dispersal (or movement) of plant pathogens is an essential component for spread of plant diseases and may occur within a field or across continents. Dispersal may be defined as the movement of propagative units of a pathogen from the original source, or focus (Campbell and Madden 1990). Mechanisms of dispersal differ widely among plant pathogens, including the following mechanisms (Campbell and Madden 1990).
- Spore ejection
- Wind
- Insect and other arthropod vectors
- Nematode vectors
- Rain
- Water runoff
- Movement of infected plant material
- Human influences such as movement of propagules in farm machinery
Dispersal and disease gradients corresponding to these mechanisms are often estimated. Dispersal gradients represent the frequency distribution of the distances traveled by all individuals in a population and the application of dispersal gradients has been useful for characterizing unidirectional dispersal (Nathan
et al. 2003). A key concept for increasing our understanding of dispersal is the difference between dispersal (inoculum) gradients and disease gradients. Inoculum gradients describe movement of the propagative unit, where host availability is not necessarily required. Disease gradients take into account all events leading to the spread of disease, including release of inoculum, transport, and deposition, as well as the presence of susceptible hosts in a disease-conducive environment.
For plant pathogens, the primary sources of inoculum are often one of three general types: point, line, or area. A point source typically has a diameter smaller than 1% of the gradient length (Campbell and Madden 1990; Zadoks and Schein 1979), while line or area sources do not necessarily have a fixed size. For example, a line source may be a row of diseased plants, and pathogen/disease measures are made at increasing distances away from this source.
Furthermore, there are two types of propagative dispersal gradients that need consideration: primary and secondary (Campbell and Madden 1990; Gregory 1968). Primary disease gradients indicate the dispersal potential of a pathogen (inoculum) in a single disease cycle (single source). Secondary disease gradients occur when inoculum is moved from lesions (plants) that had been infected during the primary dispersal event. In the following
case studies, both primary and secondary gradients will be illustrated.
In addition to measurements of dispersal within fields, long distance dispersal (LDD) is important for many plant pathogens (e.g., Asian soybean rust, tobacco blue mold). Successful transmission of plant diseases over long distances often depends on the following:
- the reproductive rate of the pathogen;
- the carrying capacity of the source locality;
- the role of atmospheric turbulence, stability, and wind speed; and
- the survival of spores during exposure to inhospitable temperature and humidity and to UVB radiation from the sun.
While many spores may be killed during atmospheric transport, a sufficient number often remain viable to cause new infections and epidemics (Campbell and Madden 1990).
In this document we introduce concepts about dispersal for different types of plant pathogens. Four case studies are introduced that emphasize the following concepts:
- primary disease gradients;
- secondary disease gradients;
- effects of droplet size and number on dispersal; and
- dispersal in large field studies.
Our analysis and illustration of the dispersal process use
the statistical package R, introduced in an associated set of exercises.
Dispersal of Bacteria
Plant pathogenic bacteria may be dispersed via several mechanisms: rain, wind, contaminated/infected seed, insects, and contaminated farm equipments (Quinn
et al. 1980). Splash dispersal is an important mechanism for short distance dispersal, typically across distances less than one meter. Splash-dispersed spores or bacteria are produced in mucilage and it is this mucilage that makes them stick to the plant surface, thereby reducing the role of wind dispersal. Several factors influence splash dispersal: inoculum concentration at the source, orientation and surface characteristics of the source, and size and velocity of raindrops. Dispersal effectiveness (i.e., distance traveled) depends on the momentum of the falling raindrops and larger rain drops are found to be more effective. Splash dispersal may also occur via overhead irrigation, such as sprinkler irrigation. Drips from the canopies saturated with rain, fog, mist, and dew have the same effect, and to some extent, cause vertical dispersal of bacteria (Fitt
et al. 1989).
Xanthomonas campestris pv.
malvacearum (causal agent of angular leaf spot of cotton) and
Erwinia carotovora subsp.
atroseptica (black leg of potato) are two examples of splash-dispersed bacteria.
For LDD, wind is the primary mechanism, mainly for dry inoculum. Dry dispersed pathogens produce lighter and smaller dispersal structures that can easily become airborne (Gregory
et al. 1959). For example, wind plays a major role in the dispersal of
Xanthomonas axonopodis pv.
citri, causing citrus canker. Furthermore, more than fifty different plant pathogenic bacteria are found to be dispersed through infested seeds (Nino-Liu
et al. 2006). One example is
Pseudomonas syringae pv.
phaseolicola, causing halo blight of bean. Five infected seeds out of ten thousand can cause an epidemic (Trigalet and Biduad 1978). Lastly, insects like honeybees are important dispersal agents of bacterial pathogens affecting fruits and flowers, such as
Erwinia amylovora, causing fire blight of pome fruits.
Dispersal of Fungi
Fungi have a wide array of dispersal mechanisms and dispersal gradients. For example, plant pathogenic fungi that associate only with plant roots (soil-borne) have relatively short dispersal gradients compared to fungi that associate with plant foliage and flowers. Also, the spore type greatly influences dispersal distance. Spores of fungi that are produced on aerial parts of a plant, such as flowers or leaves, can be dispersed easily and over a wide range of distances. Fungi which colonize the plant vascular system rely on vectors for dispersal and so do not disperse as readily. The dispersal of these pathogens depends on the range of the vector. Soilborne fungi typically disperse very slowly (Agrios 2004).
For the majority of plant pathogenic fungi, dispersal is dependent on factors such as wind, water, birds, insects, other animal, and humans, with the primary dispersal progagules being spores (Agrios 2004). Fragments of hyphae and sclerotia can also be disseminated, although that is not as common (Agrios 2004). Release mechanisms may be triggered by environmental factors, including changes in irradiance, air temperature, and relative humidity (Aylor 1990).
Spores can be actively discharged into the air or released by strong winds or light breezes and can travel distances ranging from a few centimeters to a few kilometers (Agrios 2004). For example, Rambert
et al. (1998) found that the wind speed for spore dispersal differed between leaf rust and stripe rust. For leaf rust, spores were not released until the wind speed reached 2.8 m s-1, while for stripe rust, spores were released when wind speeds were 2.3 m s-1 or greater. Lacey (1996) described three steps for fungal spore movement: take-off, dispersal, and deposition. Take-off involved spore release from a diseased plant, followed by dispersal to a different location, where finally deposition enabled the landing and subsequent infection of a new plant.
Rain is also important in spore dispersal, as water drops can cause passive spore removal when contacting a diseased leaf (Geagea
et al. 1999). Rainfall has been shown to enhance spore removal in some species infected with rust (Geagea
et al. 1999) and stripe rust occurrence was closely associated with the amount of rainfall recorded in field studies (Park 1990). Spores released as a result of water drops do not travel as far as spores released in the wind. Madden (1997) found that transport distance was usually less than 15 cm for each splash event. In addition to its role in dispersal, rain also provides an environment conducive to infection for many fungi.
Dispersal of Viruses
Viruses differ from most other pathogens in that they cannot penetrate an intact plant cuticle and cellulose found in the cell wall (Hull 2002). Viruses overcome this problem by utilizing methods of transmission that bypass the need to penetrate the outer surface of a plant, such as seed transmission or vegetative propagation, or by penetrating through a wound in the plant, such as through mechanical or insect transmission. Virus transmission by insects involves interactions among the virus, vector and the host plant. The two most important phyla in the transmission of viruses are Arthropoda and Nemata. Viruses can be taken up in one of two ways. Circulative viruses are taken up internally within the vector organism and pass through the vector's interior. Non-circulative viruses are taken up externally and these viruses do not pass through the vector's interior.
Mechanical transmission involves the introduction of the virus into a wound made on the surface of the plant. When using mechanical transmission of viruses in experiments, the goal is to make many small wounds on a plant surface without causing the death of plant cells (Hull 2002). This allows successful entry of the virus particles into the plant which will facilitate viral infection. The most common method for mechanical inoculation of plants is use of abrasives (Corbett and Sisler 1964), which are rubbed on the surface of the plant causing small wounds in the tissue. Other less common methods include spraying virus inoculum or pricking the epidermis of the plant and injecting the virus (Corbett and Sisler 1964). Viruses can also be transmitted by fungi, infected seed and infected pollen. A pollinating insect can spread infected pollen to a new host, introducing the virus to new plants. Self-pollination will result in more infected seed than cross pollination between a healthy plant and an infected plant (Corbett and Sisler 1964). Certain species of fungus-like organisms, typically Plasmodiophoromycetes or Chytridiomycetes, are vectors for some viruses (Hull 2002). Polymyxa species are important as vectors for such viruses as beet necrotic yellow vein virus (BNYVV) and wheat soilborne mosaic virus (WSBMV).
Dispersal of Nematodes
Soilborne nematodes move in the films of water that cling to soil particles. Nematode populations are generally denser and more prevalent in the world's warmer regions, where longer growing seasons extend feeding periods and increase reproductive rates. Light, sandy soils generally harbor larger populations of plant-parasitic nematodes than clay soils. Nematodes in sandy soil benefit from the more efficient aeration, the presence of fewer competitors and prey, and greater ease of movement through the root zone. Also, plants growing in readily drained soils are more likely to suffer from intermittent drought, and are thus more vulnerable to damage by parasitic nematodes. Desert valleys and tropical sandy soils are particularly challenged by nematode overpopulation (Dropkin 1980). Dispersal of nematodes is primarily through: running water (rain, irrigation, run-off, etc), and the movement of human beings, farm equipment, soil debris and plant material.
Preventing nematodes from entering uninfested areas is one way to avoid problems. Many nematodes may only spread on the order of a few feet per year without human dispersal through mechanisms such as cultivation. The following practices may help minimize nematode dispersal:
- use of certified planting material;
- soil-less growing media in greenhouses;
- cleaning soil from equipment before moving between fields (washing equipment, including tires, with water is most effective);
- keeping excess irrigation water in a holding pond so that any nematodes present can settle out, pumping water from near the surface of the pond, and planning irrigation to minimize excess water;
- preventing or reducing animal movement from infested to uninfested fields;
- composting manure to kill any nematodes that might be present, before applying it to fields (Kodira and Westerdahl 1995), and eliminating important weed hosts, such as crabgrass, ragweed, and cocklebur (Yepsen 1984).
Why Model Dispersal?
Dispersal processes underlie the development of disease foci. Information about the form of dispersal gradients is an essential component of spatially explicit epidemiological models (Roche
et al. 1995). Dispersal models can provide insights into the mechanism of inoculum dispersal and deposition, the source of inoculum, and the physical processes underlying dispersal. Developing an accurate dispersal model, however, requires a more complete understanding of the pathogen, its life cycle, spore or other propagule characteristics, the agents of dispersal and the interaction between the propagules and the environment. There are two common methods for analyzing dispersal gradients: empirical models and physical models (Campbell and Madden 1990). For the development of empirical models, researchers start with data sets and estimate parameters to fit equations that describe the probability of dispersal as a function of distance. For the development of physical models, researchers start with theories based on physical laws describing the aerodynamic and other properties of pathogen propagules, and attempt to model the dispersal events mathematically. While physical models enable a “more complete understanding of the dispersal event (canopy escape, liftoff and ascent, transport, descent and landing, impact)” (Isard
et al. 2005), the mathematical complexity and difficulty in obtaining all required model components often limit their direct application for many plant pathosystems. For an interesting example of how models might be applied for long distance dispersal, see Aylor (2003).
Some of the same models used to describe
disease progress over time can be used to study disease dispersal gradients. The significant difference between the two applications is that for disease progress curves disease intensity tends to increase with increasing time, while pathogen dispersal and disease intensity tend to decrease with increasing distance from the source of inoculum.
The dispersal model most appropriate for describing the dispersal of a particular plant pathogen depends on that pathogen’s dispersal mechanism. A researcher might try several different types of models to describe data from a given experiment or observational study. The form of the model that fits best may provide insight into the probable dispersal mechanism.
The following table presents some common dispersal models, as adapted from Campbell and Madden (1990).
Table 1. Common empirical dispersal models used to study plant pathogen dispersal.
These equations involve the following notation:
y=y(s) may represent the concentration of inoculum, disease severity, or probability of infection at a point
s units away from the source of infection. In the disease gradient context, the value 1-y represents the proportion of healthy host tissue.
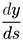
, accordingly, represents the rate at which either the inoculum concentration, disease severity, or probability of infection changes as the distance away from the source changes.
b is the rate parameter determining how steep the disease gradient is (a greater value for
|b| leads to a steeper disease gradient). When
b has no units (Models B, E, F) it is more difficult to compare two models, as they might have different distance scales.
The parameter
a in some ways acts like an initial condition. In the event that
y represents a probability,
a will be chosen so that the integral of the probability of dispersal over the range of potential distances from the source is 1.
Note that in models A, C, and D (the models in which
b has units) it is assumed that the rate of change in dispersal with distance from the source is the same across all distances.
In each equation,
y is nonlinear in terms of
s, so
nonlinear regression can be used to estimate the parameters. Another option, which is simpler in some cases, is to linearize the equations (the resulting forms are listed in the table) and then use
linear regression to estimate the parameters.
Models A and B, the exponential law and the power law respectively, are the simplest models presented here, and are both commonly applied. In the more general version of the exponential law, the
s in the integrated form can be raised to some power. In many cases where one of these models provides a good fit to data, the other may fit almost as well.
One difference between the exponential and power law models is the aforementioned pitfall of
b having no units in the power law model, a pitfall that is avoided in the exponential model. Another difference is that at the source, the power law model gives a disease intensity of
∞, which is unrealistic, while the exponential model gives a finite density that can be controlled through the parameters. However, at a very small distance from the source, the exponential model does not necessarily reflect the high inoculum density, while the power law model will generally give the necessarily large values. As a generalization, when pathogens are dispersed by splashing water, the exponential model may be a better fit, and when wind-dispersed pathogens are very small (smaller than 10 μm) the power law model may be more appropriate (Campbell and Madden 1990).
The remaining models C-F take into account the amount of healthy host tissue. Equation C is analogous to the monomolecular model used in
studying disease progress over time. Models C and D assume that the rate of change in dispersal is constant over different distances, while E and F do not. However, C and D have units for the parameter
b (making it easier to compare models fit to different data sets) while E and F do not. C and E are functions of the amount of healthy tissue remaining, while D and F are functions of both the amount of diseased tissue remaining and the amount of healthy tissue remaining, based on linearized dispersal models where the response is
ln(1/1-y)).
A specific form of equation F which has been applied in epidemiology is the Cauchy model: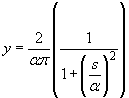 , where
α is the median dispersal (Shaw 1997).
, where
α is the median dispersal (Shaw 1997).
Next we use the R programming environment, introduced in a
companion set of exercises (Garrett
et al. 2007) to illustrate how changing parameters changes the shape of these models, as well as illustrating the differences between models A through F. First consider the shapes for the exponential and power law models. The following R code produces a function that will plot the two curves with parameters a1 and b1 corresponding to the power law model and parameters a2 and b2 corresponding to the exponential model. This new function incorporates an existing R function,
curve; for more information about this function, enter
help(curve) on the R command line. For more information about writing functions in R, see
Garrett
et al. (2007).
plot.exp.power
<-
function(a1,a2,b1,b2,max1,max2)
{
curve(a1*x^(-b1),
from=0,
to=max1,
add=FALSE,
lty=1,
xlab='Distance (m) from
source',
ylab='Disease incidence',
col='black',
xlim=c(0,60),
ylim=c(0,50))
title(main='Power law (black)
and Exponential (red)')
curve(a2*exp(-b2*x),
from=0,
to=max2,
add=TRUE,
lty=1,
col='red')
}To explore differences in these two curves, plots may be made applying the function with parameter estimates from a particular example, such as the parameters obtained from research on wheat stripe rust in Hermiston, Oregon in 2002 (Sackett and Mundt 2005),
Case Study #4.
plot.exp.power(a1=184.9,
a2=18.59,
b1=2.07,
b2=0.106,
max1=60,
max2=60)
The resulting graph looks like:
Output
Click on the image for larger version.
If parameters are modified as:
plot.exp.power(a1=113.9,
a2=11.49,
b1=2.07,
b2=0.106,
max1=60,
max2=60)
the resulting graph has a less steep dispersal gradient:
Output
Click on the image for larger version.
To explore models C and D, a similar plot function may be defined as for models A and B. As an example:
plot.C.D
<-function(a1,a2,b1,b2,max1,max2){
curve(1-a1*exp(b1*x),
from=0,
to=max1,
add=FALSE,
lty=1,
xlab='Distance (m) from source',
ylab='Disease incidence',
col='black',
xlim=c(0,60),
ylim=c(0,1))
title(main='Model C (black) and Model D (red)')
curve(1/(1+a2*exp(b2*x)),
from=0,
to=max2,
add=TRUE,
lty=1,
col='red')
}which, when applied using the following parameter values:
plot.C.D(a1=0.03,
a2=5.59,
b1=0.08,
b2=0.106,
max1=60,
max2=60)
returns:
Output
Click on the image for larger version.
Likewise, for models E and F:
plot.E.F <-
function(a1,a2,b1,b2,max1,max2){
curve(1-a1*x^(b1),
from=0,
to=max1,
add=FALSE,
lty=1,
xlab='Distance (m) from source',
ylab='Disease incidence',
col='black',
xlim=c(0,60),
ylim=c(0,1))
title(main='Model E (black) and Model F (red)')
curve(1/(1+a2*x^b2),
from=0,
to=max2,
add=TRUE,
lty=1,
col='red')
}The following plot may be observed with the specified parameters:
plot.E.F(a1=0.09,
a2=2,
b1=0.6,
b2=0.6,
max1=60,
max2=60)
Output
Click on the image for larger version.
Suggested Exercise
Using the R functions applied above, examine how different parameter estimates for all the different models modify the shape of the dispersal curves. Also, modify the maximum dispersal distances plotted (max1 and
max2 in the functions) and see what this shows about the gradients fit by the different models.
Next, primary disease gradients of bacteria
